40 hydrogen energy level diagram
An energy level diagram is a diagram that shows the energies of the reactants, the transition state(s) and the products of the reaction with time; The transition state is a stage during the reaction at which chemical bonds are partially broken and formed Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, : Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the ...
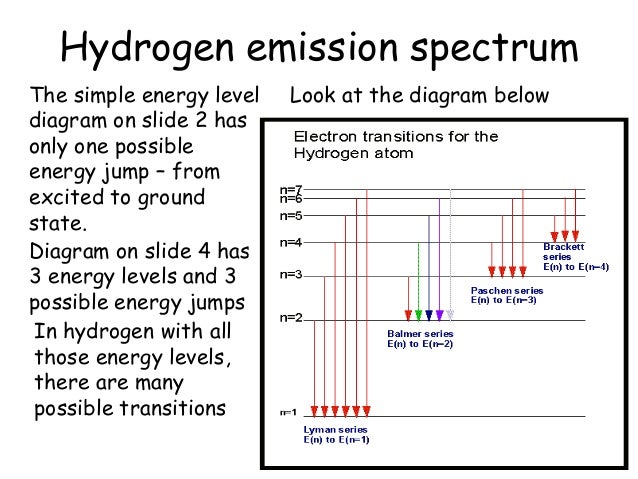
Observe the following diagram showing electronic transition in the hydrogen spectrum. i. Electron jumps from higher energy level to n = 1. Which series does it correspond to? ii. Electron jumps from higher energy level to n = 4. Which series does it correspond to? iii. Which transition will give second line of Balmer series?

Hydrogen energy level diagram
The emission spectrum of hydrogen Energy levels of the hydrogen atom: De-excitation of electron results in emission of photon-13.6 eV 0.0 eV E PHYS 1493/1494/2699: Exp. 7 - Spectrum of the Hydrogen Atom Energy level diagrams for IONS Atoms with 1, 2, or 3 valence electrons lose them to form 1+, 2+ or 3+ ions respectively. naming metallic ions - the full name of the atom is followed by the word ion. Mg2+ is the magnesium ion Group 1 (1+) (lose 1e) Group 2 (2+) (lose 2e) Group 13 (3+) (lose 3e) Energy level diagrams for IONS What is energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is called the "K shell" followed by the "L shell" then the "M shell" and so on away from the nucleus. The shells can be denoted by alphabets (K, L ...
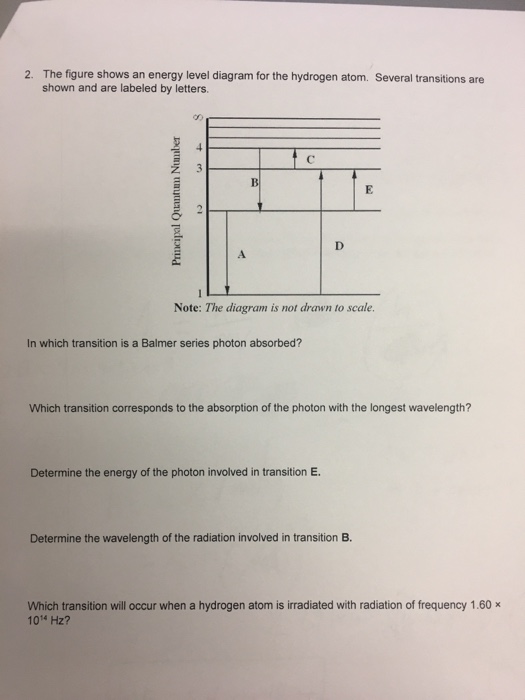
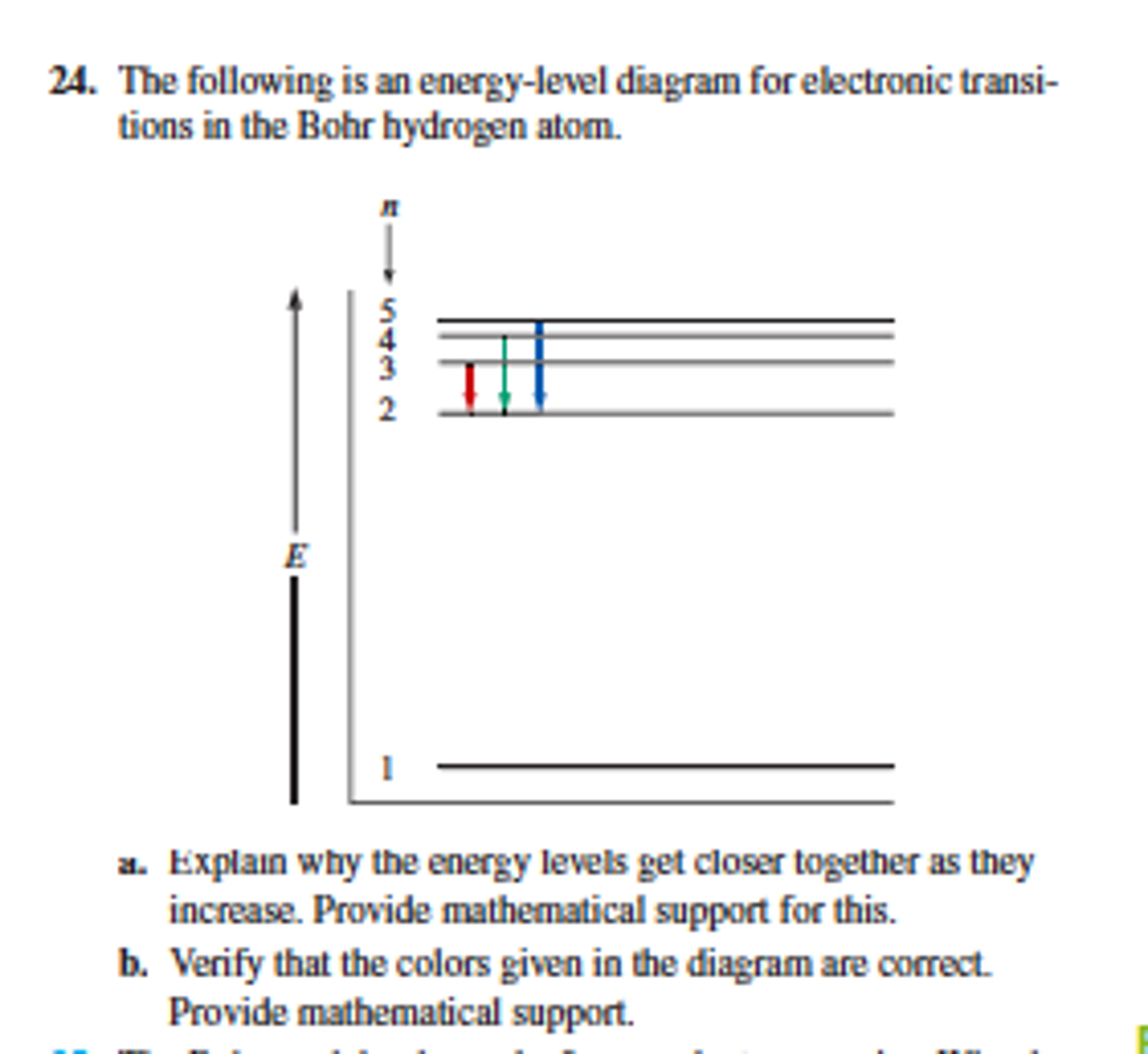
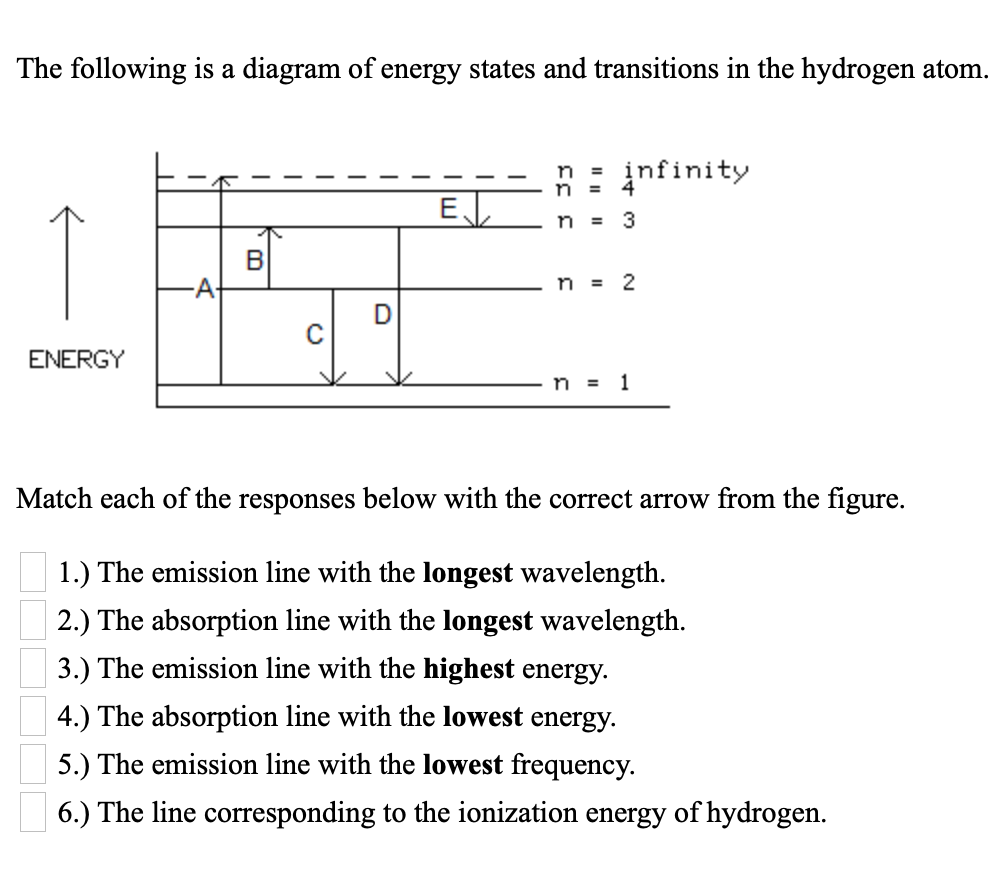
Hydrogen energy level diagram. The energy levels agree with the earlier Bohr model, and agree with experiment within a small fraction of an electron volt. If you look at the hydrogen energy levels at extremely high resolution, you do find evidence of some other small effects on the energy. The 2p level is split into a pair of lines by the spin-orbit effect. One method of storing wind energy is the production of hydrogen through the electrolysis of water.This hydrogen is subsequently used to generate electricity during periods when demand can not be matched by wind alone. The energy in the stored hydrogen can be converted into electrical power through fuel cell technology or a combustion engine linked to an electrical … Hydrogen And Oxygen Energy Level Diagram Redox Water And Energy Burning Fossil Fuels. TERMS IN THIS SET (33) When hydrogen and oxygen react they produce water and energy The type of reaction that occurs when hydrogen and oxygen react is an exothermic reaction (releases energy) Which of the transitions in the hydrogen atom energy-level diagram shown here requires the absorption of the longest wavelength photon? transition a b. ransition b c transition c d. ransition d n 5 a. e. None of these are correct, because photons are emitted, not absorbed, by these transitions 15.
The energy from the Sun - both heat and light energy - originates from a nuclear fusion process that is occurring inside the core of the Sun.The specific type of fusion that occurs inside of the Sun is known as proton-proton fusion.. Inside the Sun, this process begins with protons (which is simply a lone hydrogen nucleus) and through a series of steps, these protons fuse together … Millions of thanks from depths of My Heart to every subscriber and Visitor.Now you can post your confusions in video format. Make a Video of your own describ... If an electron is in the first energy level, it must have exactly -13.6 eV of energy. If it is in the second energy level, it must have -3.4 eV of energy. An electron in a hydrogen atom cannot have -9 eV, -8 eV or any other value in between. Let's say the electron wants to jump from the first energy level, n = 1, to the second energy level n = 2. Hydrogen has signature colors that appear when the atoms are excited. This image from NASA-Ames shows a hydrogen arc lamp fluorescing: Neils Bohr used the emission spectra of hydrogen to develop his model of the atom. In short, quantized energy levels release specific bands of light with unique colors.
The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV. Hint: Energy level diagram is the direct consequence of the principal quantum number - 'n'. Energy diagram is necessary to determine the energy difference between two states and explain the hydrogen spectrum. Complete step-by-step answer: The following diagram shows the energy level diagram of the Hydrogen atom. Hydrogen Energy Level Diagram. New Resources. Open Middle: Distance, Midpoint, Slope (3) Open Middle: Build a Trig Equation (3) Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from ...
Seven electrons are trapped in a one-dimensional infinite potential well of width L.What multiple of 8 m L 2 h 2 gives the energy of (a) the first excited state, (b) the second excited state, and (c) the third excited state of the system of seven elec-trons? (d) Construct an energy-level diagram for the lowest four energy levels of the system.
Energy Level Diagram February 17, 2017 By Mrs Shilpi Nagpal Leave a Comment Energies of orbitals of hydrogen and hydrogen like particles depend upon the value of principal quantum (n) number only , those of multi-electron atoms depend both upon principal quantum number( n ) as well as azimuthal quantum number(l).
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
level diagram, or potential energy profile, as shown in Figure 13.1. The vertical axis gives the potential energy for the reaction, while the horizontal axis is a relative (i.e., time) scale that shows the progress of the reaction. The diagram indicates that there is a "hill" or energy barrier that needs to be overcome before any products ...
Einstein's theory for the photoelectric effect, where a photon has energy proportional to its frequency, Bohr postulated the existence of energy levels in the atom. He assumed that the energy associated with the photons of light were the result of transitions in the atom from one level to another, with the energy of the photon being equal to the
Energy level diagram . The energy of the electron in the n th orbit of the hydrogen atom is given by, En = -13.6 /n 2 eV. Energy associated with the first orbit of the hydrogen atom is, E 1 = -13.6 /1 2 = -13.6 eV. It is called ground state energy of the hydrogen atom. Energy associated with the second orbit is given by, E 2 = -13.6 /2 2 = -3.4 eV
Download scientific diagram | The evolution of the a-parameter with temperature for some energy levels of the hydrogen atom according to Eq. (26) from publication: Non-Gaussian effects of the Saha ...
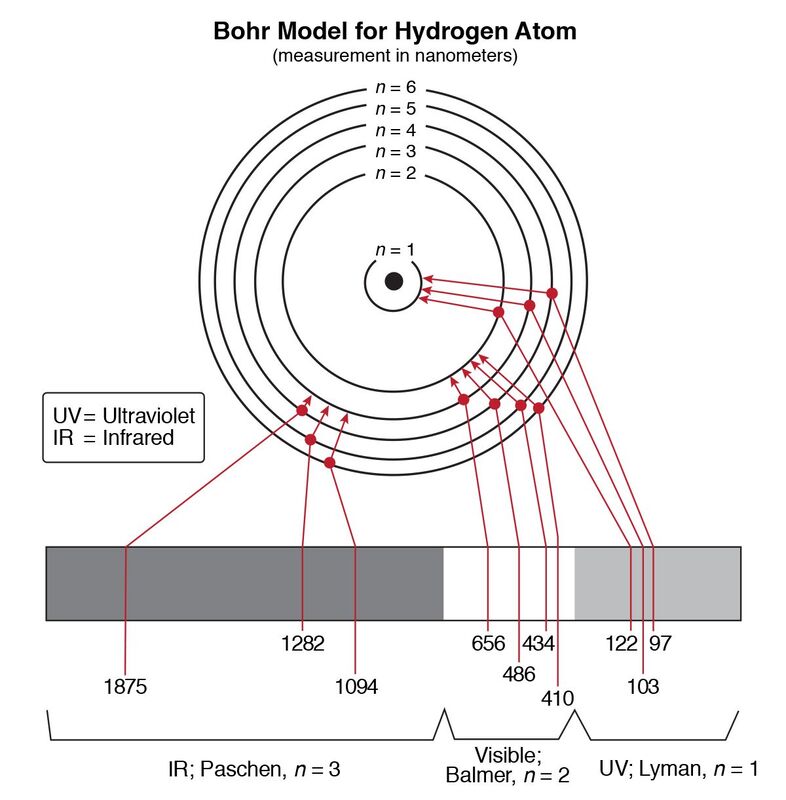
Nov 10, 2021 · The hydrogen spectrum lines are: Lyman series, Balmer series, Paschen series, Brackett series, Pfund series. The infinity level indicates the maximum amount of energy that an electron can have as a component of a hydrogen atom. If the electron's energy reaches that threshold, it is no longer a part of the atom.
Hydrogen sulfide is a chemical compound with the formula H 2 S.It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The underground mine gas term for foul-smelling hydrogen sulfide-rich gas mixtures is stinkdamp, the suffix deriving from an …
The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon.
Atomic Energy Levels and Grotrian Diagrams, Volume I: Hydrogen I - Phosphorus XV presents diagrams of various elements that show their energy level and electronic transitions. The book covers the first 15 elements according to their atomic number. The text will be of great use to researchers and practitioners of fields such as astrophysics that ...
2. Draw and label an energy level diagram for the hydrogen atom. In your diagram show how the series of lines in the ultraviolet and visible regions of its emission spectrum are produced, clearly labelling each series. (Total 4 marks) 3. Describe the emission spectrum of hydrogen. Outline how this spectrum is related to the energy levels in the ...
Energy level for Hydrogen image credit :Ivan Ramirez, Energy-Level Diagrams for Hydrogen, CC BY 4.0 What is the energy level diagram? Energy level means of evaluating the amount of energy electron may require and will be released during the transition, if they jump from one orbit to other.
Imgur. The energy of the electron of a monoelectronic atom depends only on which shell the electron orbits in. The energy level of the electron of a hydrogen atom is given by the following formula, where. n. n n denotes the principal quantum number: E n = − 1312 n 2 kJ/mol. E_n=-\frac {1312} {n^2}\text { kJ/mol}. E n.
Hydrogen Spectrum - Wavelength, Diagram, Hydrogen Emission Spectrum. Hydrogen Spectrum introduction. We all know that electrons in an atom or a molecule absorb energy and get excited, they jump from a lower energy level to a higher energy level, and they emit radiation when they come back to their original states.

Marina City Theater, Chicago, Illinois, Roof and Partial Concrete Frame Development Drawing (1961-1962) // Bertrand Goldberg American, 1913-1997
What is energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is called the "K shell" followed by the "L shell" then the "M shell" and so on away from the nucleus. The shells can be denoted by alphabets (K, L ...
Energy level diagrams for IONS Atoms with 1, 2, or 3 valence electrons lose them to form 1+, 2+ or 3+ ions respectively. naming metallic ions - the full name of the atom is followed by the word ion. Mg2+ is the magnesium ion Group 1 (1+) (lose 1e) Group 2 (2+) (lose 2e) Group 13 (3+) (lose 3e) Energy level diagrams for IONS
The emission spectrum of hydrogen Energy levels of the hydrogen atom: De-excitation of electron results in emission of photon-13.6 eV 0.0 eV E PHYS 1493/1494/2699: Exp. 7 - Spectrum of the Hydrogen Atom

























0 Response to "40 hydrogen energy level diagram"
Post a Comment