38 ethylene molecular orbital diagram
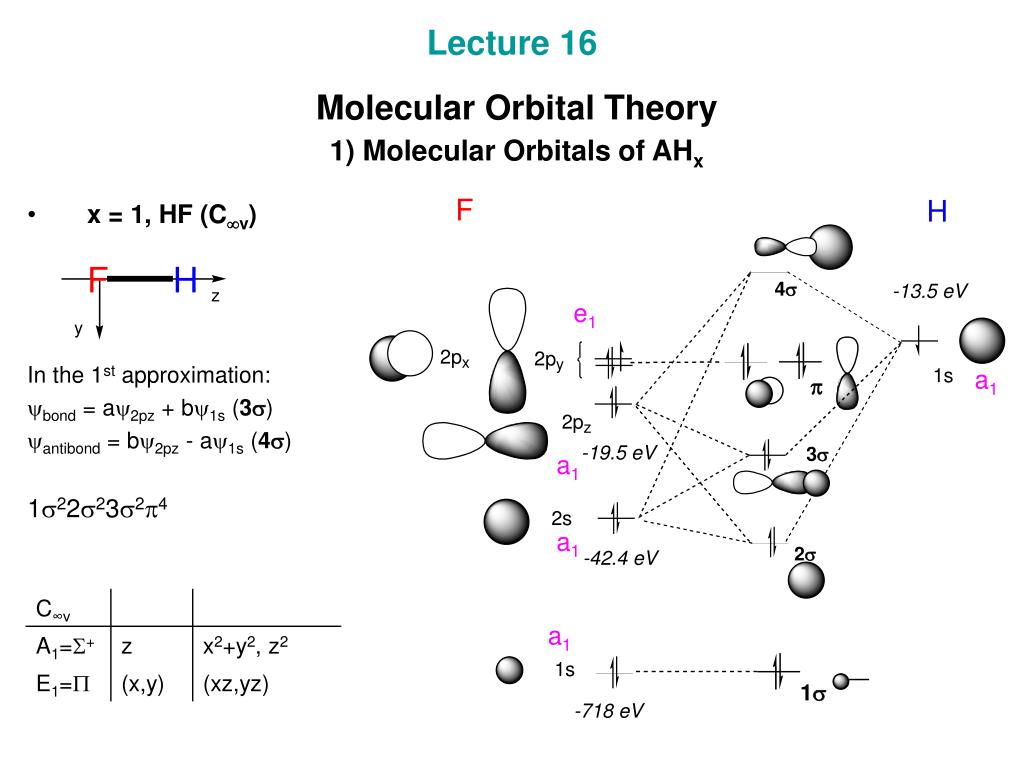
Molecular Orbital Theory. Bonding and Antibonding Molecular Orbitals. In molecular orbital theory, bond order is also defined as the difference, divided by two, between the number of bonding and antibonding electrons; this often, but not always, yields the same result. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding. For heteroatomic dinuclear molecule, energy of BMO closely matches with those of more electronegative atom & energy of ABMO closely matches with those of less electronegative atom.
Molecular orbital theory was put forward by Hund and Mullikan in 1932. This theory is modern and more rational. This theory assume that in molecules, atomic orbitals lose their identity and the electrons in molecules are present in new orbitals called molecular orbitals.
Ethylene molecular orbital diagram
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Figure 10. The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and antibonding electrons. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory. Valence Bond Theory proposes that electrons are localized between two atoms. • Standard bonding picture for ethylene is viewed as being made from 2 sp2 hybridized C atoms, and consists of a C-C double bond. •. MOT does not employ hybridization and does not assume bonding arrangements. •. Build ethylene from two CH2 groups without preconceived bonding arrangements. •
Ethylene molecular orbital diagram. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. Chad explains how to draw the Bonding and Antibonding Pi Molecular Orbitals for 1,3,5-hexatriene identifying both the HOMO and LUMO. It should also be pointed out that these vertical nodes are always arranged symmetrically in the molecular orbital diagrams. FIGURE 5.15 Molecular orbitals for ethylene. Promotion of an electron from the ground state to the excited state is known as a n - n transition and is usually In the following diagram, we see that the bonding v molecular orbital for ethylene has no nodes perpendicular to the bond axis, whereas the... Molecular Orbital Theory. I'm having a lot of trouble with this stuff. I don't really know how to start these questions (such as how to draw a correlation So here, I basically ask, how do I draw a correlation diagram? Like, how do I know how many electrons to put in the bonding atomic orbital and...
Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12. The molecular orbital theory is one of the most productive models of chemical bonding. It is the basis of quantitative calculations, including those regarding the Molecular orbital theory in overall involves a lot of complicated mathematics. However, the fundamental ideas behind the theory are very easy to... The overall molecular orbital energy level diagram of CO2 is shown in Figure 2-7. The molecular orbital picture of other linear triatomic species, such. As for ethylene and π-allyl, the 2p orbitals of the carbon atoms in the chain may interact in a variety of ways, with the lowest energy π molecular... A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
Similarly, the molecular orbital diagrams for homonuclear diatomic compounds of the alkaline earth metals (such as Be2), in which each metal atom has Use a qualitative molecular orbital energy-level diagram to predict the valence electron configuration, bond order, and likely existence of the Na2− ion. Notes on molecular orbital calculations. First printing, 1961 Second printing, with stretching vibrations of ethylene in the infrared a r e different f r o m those of acetylene and ethane. IN THE APPLICATION of molecular orbital theory to calculations of chemical binding energies, we... The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive... The molecular orbital theory is a concept of quantum mechanics where atomic linearly combines to form molecular orbitals and we describe the wave nature of atomic particles. The above diagram shows the Molecular Orbital(MO) diagram of ethene/ethylene.
Hello! Just finished a lecture in my ochem class and was going through the notes and had some questions if yall don't mind. I am already watching some youtube videos on molecular diagram and orbitals because I have no clue whats going on there but if you guys could please help out that would be great! * **First starting off with conjugation and energy, here is a pic: https://imgur.com/lmg6PDn** Does higher energy = less stable? So the first one monoene has a higher energy than the two dienes c...
The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. Placing an electron in this orbital therefore stabilizes the H2 molecule. This diagram suggests that the energy of an H2 molecule is lower than that of a pair of isolated atoms.
Molecular orbital diagrams provide qualitative information about the structure and stability of the electrons in a molecule. This article explains how to create molecular orbital diagrams in LaTeX by means of the package MOdiagram.
• Molecule orbital theory (Robert Mullikan). • Electrons are delocalised - Different to Lewis and hybridisation (these are not MO). • Energy level diagram represents this interaction. - Two s orbitals interaction to create a low energy bonding and high energy anti-bonding molecular orbital.
Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Hello! Just finished a lecture in my ochem class and was going through the notes and had some questions if yall don't mind. I am already watching some youtube videos on molecular diagram and orbitals because I have no clue whats going on there but if you guys could please help out that would be great! * **First starting off with conjugation and energy, here is a pic: https://imgur.com/lmg6PDn** Does higher energy = less stable? So the first one monoene has a higher energy than the two dienes c...
● Atomic Orbital - region (volume) in space where probability of finding an electron (of a. specific energy) is very high (90 - 95 %), NO FIXED ○ Amplitude, ψ (+ or -) = orbital phase; Probability, ψ 2 = orbital shape. ○ P-orbitals are not spherically symmetric (like s-orbitals); each p-orbital has a.
Ethylene is capable of acting as a ligand as the C=C π bond can donate electron density to an empty metal d orbital, forming a σ bond. View Ethylene Molecular Orbitals here. Explore Metal-Ligand bonding with other molecules.
Hello! Just finished a lecture in my ochem class and was going through the notes and had some questions if yall don't mind. I am already watching some youtube videos on molecular diagram and orbitals because I have no clue whats going on there but if you guys could please help out that would be great! * **First starting off with conjugation and energy, here is a pic: https://imgur.com/lmg6PDn** Does higher energy = less stable? So the first one monoene has a higher energy than the two dienes c...
The molecular orbital diagram for the π-molecular orbitals of butadiene as a result of combining the π-molecular orbitals of two ethene molecules. This shows .Bonding orbitals in Ethene (Ethylene) sp 2 Background: Use the buttons to display the sp 2 orbitals that make up the sigma framework and the...
2018, 20,14211. Diagrams for comprehensive molecular. orbital-based chemical reaction analyses: reactive orbital energy diagrams†. 1 An example of a frontier molecular orbital diagram: diagram of the. butadiene (C. 4. H. 6. ) + ethylene (C. 2.
Molecular Orbital Tutorial. Barry Linkletter Department of Chemistry, University of Prince Edward Island Abstract This tutorial examines a method for constructing hybrid orbitals. Page 30 of 35. Fig. 31: Molecular orbital diagram for ethylene.
Chapter 2 - Molecular Orbital Theory Big-picture: Now that we understand aspects of molecular structure, we can look in more detail at bonding - how atoms are bonded together, energetics of bonding, where electrons reside in bonds, etc.
Assuming the molecular orbital diagram in Figure 9.16 applies to BrO, write its electron configuration (where Br uses $4 s$ and $4 p$ orbitals). What is the highest occupied molecular orbital (HOMO) for the molecule? Check back soon!
• Standard bonding picture for ethylene is viewed as being made from 2 sp2 hybridized C atoms, and consists of a C-C double bond. •. MOT does not employ hybridization and does not assume bonding arrangements. •. Build ethylene from two CH2 groups without preconceived bonding arrangements. •
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory. Valence Bond Theory proposes that electrons are localized between two atoms.
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Figure 10. The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and antibonding electrons.


0 Response to "38 ethylene molecular orbital diagram"
Post a Comment