41 draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
Recitation Week 10 (test 3 - Recitation 2) - GitHub Pages Oct 24, 2016 · E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+ B) N2^2+ C) B2 Solved 6. Draw the molecular orbital diagram shown to ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B2, B, C, B and N, Molecular Orbital Diagram B B24 C2 B2 N22 Which molecule is paramagnetic? Question: 6. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
Solved Use the molecular orbital diagram shown to | Chegg.com Question: Use the molecular orbital diagram shown to determine which of the following is paramagnetic. (a) O^2-_2 (b) Ne^2+_2 (c) O^2-_2 (d) F_2+_2 (e) None ...
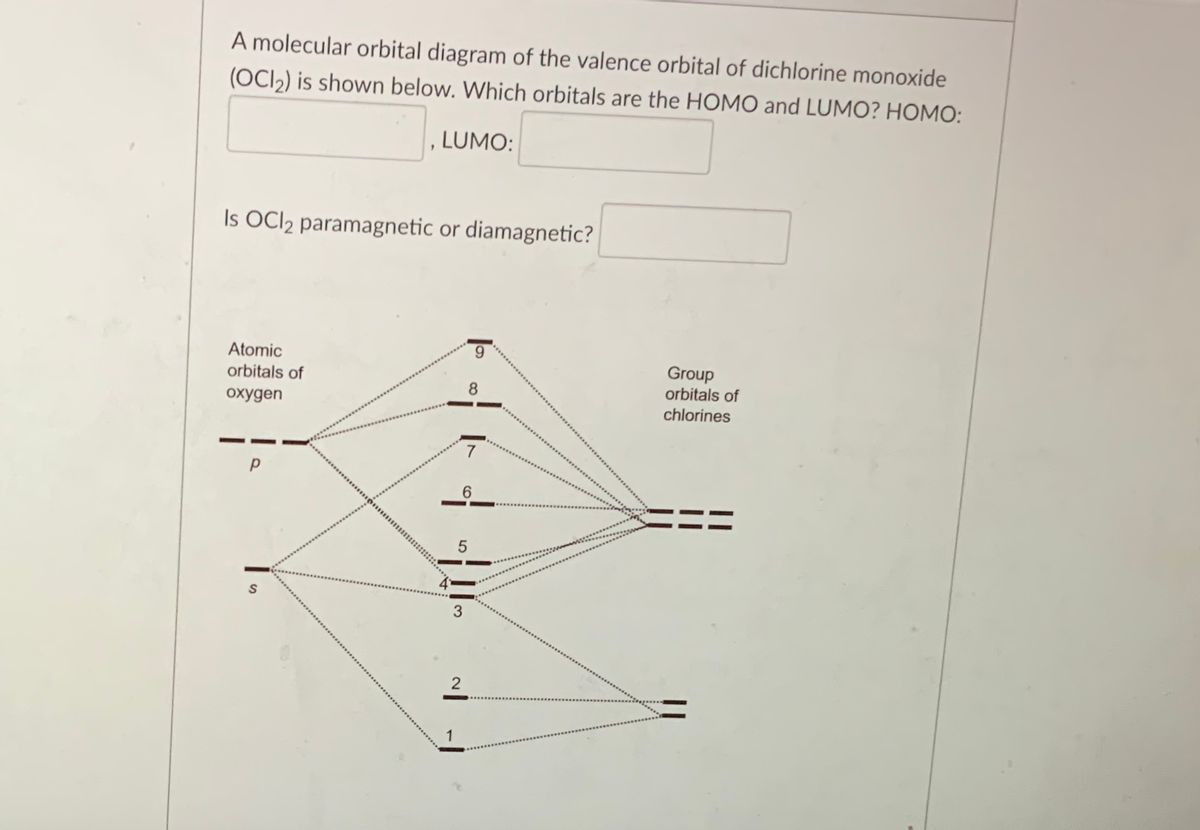
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
Draw the molecular orbital diagram shown to determine ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. N22+ B22+ B22- B2 C22- science chemistry 1 0 Add a comment Improve this question Next > < Previous Sort answers by oldest Homework Answers Recommended Answer Answer #1 4 0 Add a comment Know the answer? Add Answer to: Solved Draw the molecular orbital diagram shown to ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.Answer options:B2B22+N22+C22-B22-Question: Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.Answer options:B2B22+N22+C22-B22- C22- Molecular Orbital Diagram Answer to Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. N22+ B22+ B B2 CeV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of the lowest energy ...
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.. Use molecular orbital diagrams to predict which of the ... Use the molecular orbital diagram shown to determine which of the following is paramagnetic. asked Jul 15, 2019 in Chemistry by brittanyr9777. general-chemistry. Nitrogen can lose an electron to form N2+. Given the molecular orbital configuration of N2 [core] (σ2s)2 (σ *2s)2 (π2p)4 (σ2p)2 is N2+ diamagnetic or paramagnetic? asked Jun 30 ... Solved Draw the molecular orbital diagram shown to ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. F22+. O22-. Ne22+. O22+. None of the above are paramagnetic. Best Answer. This is the best answer based on feedback and ratings. 100% (48 ratings) Solved Use the molecular orbital diagram shown to determine Use the molecular orbital diagram shown to determine which of the following is paramagnetic. a) B2 b) C2^2- c) N2^2+ d) B2^2- e) B2^2+ Use the molecular ... is b22+ paramagnetic or diamagnetic Using molecular orbital diagrams to determine which of the following are paramagnetic e B2 B2 paramagnetic. Lack thereof 2+ has the same number of electrons as B2, and since B2 is,... Posses 2 unpaired electrons, it is paramagnetic, so is C2 2+ a molecule is paramagnetic so!
Solved Draw the molecular orbital diagram shown to ... Science. Chemistry. Chemistry questions and answers. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. O 0,2 O F₂2+ O Ne 2+ O 0₂2+ None of the above are paramagnetic. Question: Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. Pictorial Molecular Orbital Theory - Chemistry LibreTexts There are two unpaired electrons, so molecule is paramagnetic. 4.The molecular orbital diagram for a diatomic Neon molecule, Ne 2, is. Bond Order = 1/2(10 - 10) = 0; bond order is zero, so Ne 2 is unstable. diamagnetic; 5. The molecular orbital diagram for the diatomic fluorine molecule, F 2 is. B.O. = 1/2(10 - 8) = 1 a. Draw the molecular orbital diagram shown to determine ... a. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B_2^2+, B2, C_2^2-, B_2^2- and N_2^2+ b. Draw the Lewis structures and molecular orbital diagrams... Molecular Orbital Theory - Chemistry Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.
Solved Use the molecular orbital diagram shown to ... Question: Use the molecular orbital diagram shown to determine which of the following is paramagnetic. Atomic orbitals Molecular orbitals Atomic orbitals 2p 2P קני 25 02 02, F2 Nez F2 None of the ions are paramagnetic. 2+ 02 2- This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (1 rating) ;answer is p … Quiz 1 Flashcards | Quizlet Use the molecular orbital diagram shown to determine which of following is paramagnetic A) C2^2- B) B2 C) B2^2- D) N2^2+ E) B2^2+ B Based on molecular orbital theory, the bond orders of H—H bonds in H2, H2^+, and H2^- are ______, respectively 1, 1/2, 1/2 Use the molecular orbital diagram shown to determine which ... more the number of unpaired electrons, more will be the paramagnetism.the given molecular orbital diagram contains orbitals to be filled in the order: s1s, s*1s, s2s, s*2s, p2p, s2p, p*2p, s*2paccording to molecular orbital theory, the electronic configuration of c22-= s1s2, s*1s2, s2s2, s*2s2, p2p4, s2p2=> number of unpaired electrons = 0the … Answered: Draw the molecular orbital diagram… | bartleby Answered: Draw the molecular orbital diagram… | bartleby Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+, B2, C22-, B22-, and N22+ Question Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B 22+, B 2, C 22-, B 22-, and N 22+ Expert Solution
Chem Final Exam Flashcards - Quizlet Draw the best Lewis structure for BrO4⁻ and determine the formal charge on bromine. A) -1 B) +1 ... Draw the molecular orbital diagram shown to determine which of the following is most stable. A) F2 B) F22⁺ ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) O22⁻ ...
Chemical bonding Flashcards - Quizlet 59) Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) O2^2−. B) Ne2^2+. C) O2^2+. D) F2^2+. E) None of the above are paramagnetic. D) F2^2+. 60) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+.
By writing molecular orbital configuration for NO,CO,O2 ... Also see here... Bond order for "NO"^+ Order by bond length: "NO", "NO"^(+), "NO"^(-) Is "CO" a Lewis acid? "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the ...
Answered: 1 -Use the molecular orbital diagram… | bartleby Transcribed Image Text: 1 -Use the molecular orbital diagram shown to determine which of the following are paramagnetic a) F,2" b) Nez2* c) 0,2 d) 0,2 a O b O c O d O
[Answered] Use the molecular orbital diagram shown to ... Use the molecular orbital diagram shown to determine which of the following is most stable. A. F22+ B. Ne22+ C. F22- D. O22+ E. F2 - 3580227
Study Chapter 4 Pre-Exam Flashcards - Quizlet Use the molecular orbital diagram shown to determine which of the following are paramagnetic. B₂ Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
Use molecular orbital diagrams to predict which of the ... Use the molecular orbital diagram shown to determine which of the following is paramagnetic. asked Jul 15, 2019 in Chemistry by brittanyr9777. general-chemistry. Nitrogen can lose an electron to form N2+. Given the molecular orbital configuration of N2 [core] (σ2s)2 (σ *2s)2 (π2p)4 (σ2p)2 is N2+ diamagnetic or paramagnetic?
Draw the molecular orbital diagram shown to determine | Chegg.com Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. Answer options: Ne22+ O22- F22+ O22+ None of the above are ...
Solved Draw the molecular orbital diagram shown to determine Question: Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. O22+ Ne22+ F22+ O22- · This problem has been solved!
Draw the molecular orbital energy diagram for oxygen ... Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown: (i) Electronic configuration: (ii) Bond order: Here N b = 8; N a = 4 The two oxygen atoms in a molecule of oxygen are united through two covalent ...
Answered: Draw a molecular orbital diagram and… | bartleby Draw a molecular orbital diagram and use it to determine which of the following is paramagnetic. F22+ Ne22+ O22- O22+ None of the above is paramagnetic. Expert Solution Want to see the full answer? Check out a sample Q&A here See Solution Want to see the full answer? Check out a sample Q&A here See Solution star_border
chemistry 1a Chapter 10 Flashcards | Quizlet Use the molecular orbital diagram shown to determine which of the following is most stable A) Ne2^2⁺ B) F2^2⁺ C) F2^2⁻ D) F2 E) O2^2⁺ E). O2^2⁺ Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A) O2 2⁺ ...
C22- Molecular Orbital Diagram Answer to Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. N22+ B22+ B B2 CeV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of the lowest energy ...
Solved Draw the molecular orbital diagram shown to ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.Answer options:B2B22+N22+C22-B22-Question: Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.Answer options:B2B22+N22+C22-B22-
Draw the molecular orbital diagram shown to determine ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. N22+ B22+ B22- B2 C22- science chemistry 1 0 Add a comment Improve this question Next > < Previous Sort answers by oldest Homework Answers Recommended Answer Answer #1 4 0 Add a comment Know the answer? Add Answer to:

0 Response to "41 draw the molecular orbital diagram shown to determine which of the following is paramagnetic."
Post a Comment