39 transition state energy diagram
PDF Structure of the Transition State the beginning state. The energy of the TS≠ must be closer to the beginning state. There's just no other way to draw the diagram. We call this TS≠ "early" since the structure of the transition state has not evolved far from its starting point. Case 2: the ending state is higher than the beginning state - In this case Understanding Energy Diagrams - Concept - Chemistry Video ... That's the energy it takes for the reaction to occur. So it's from the reactants to the highest point on the curve in the transition state. So up here we have the transition state. And so the difference between the reactants and the transition state, as in 1, this difference, is what we call the activation energy. You might see that as E sub a.
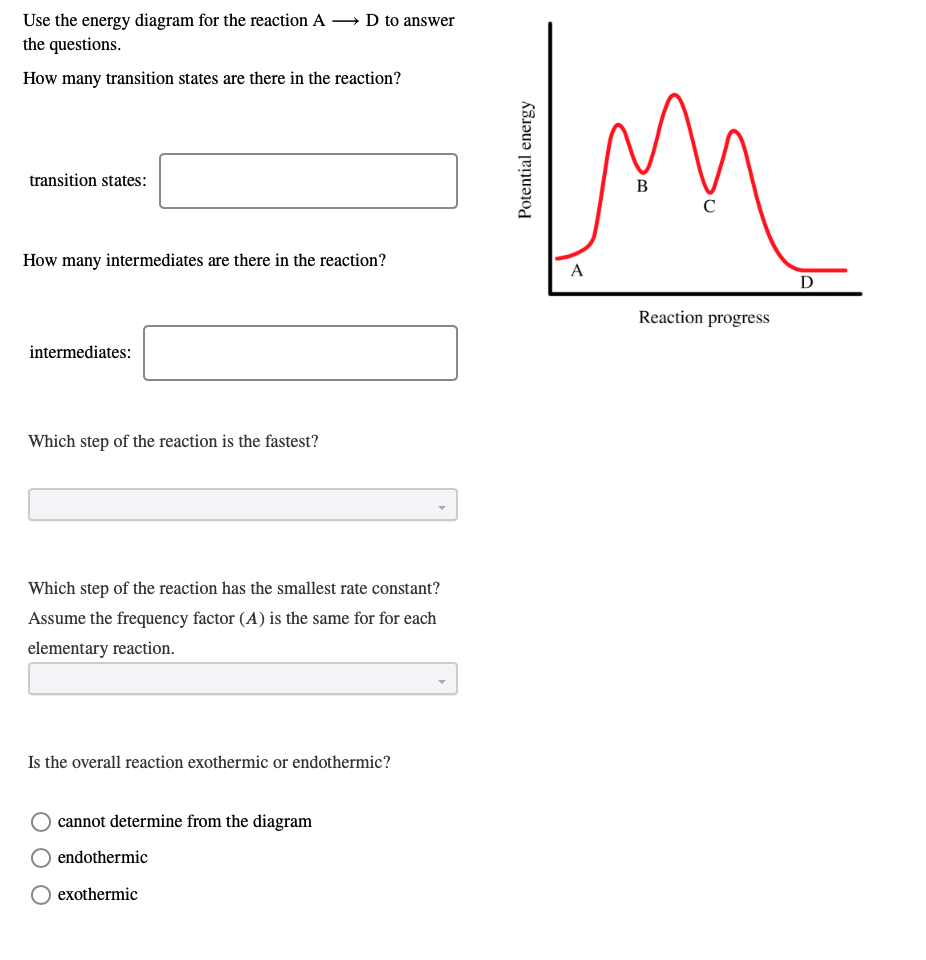
Energy Reaction Progress A B C D 22 The transition state ... 22. The transition state is found at point _____ on the diagram. 23. The products are found at point _____ on the diagram. 24. The free-energy change for the reaction is indicated at point _____ on the diagram. 25. The reactants are found at point _____ on the diagram. Consider the reaction of 2-bromo-2-methylpropane with water, shownbelow, to ...
Transition state energy diagram
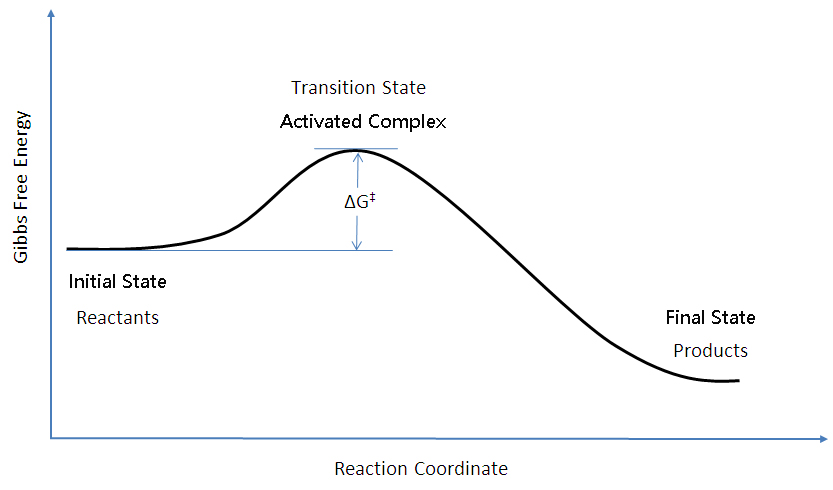
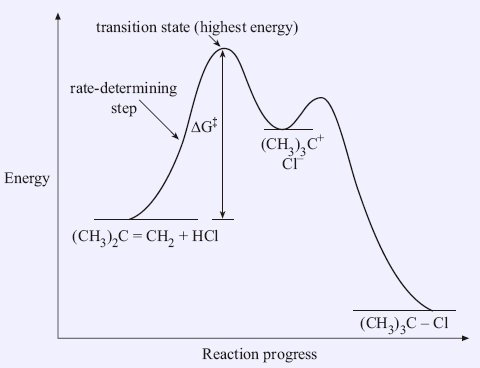
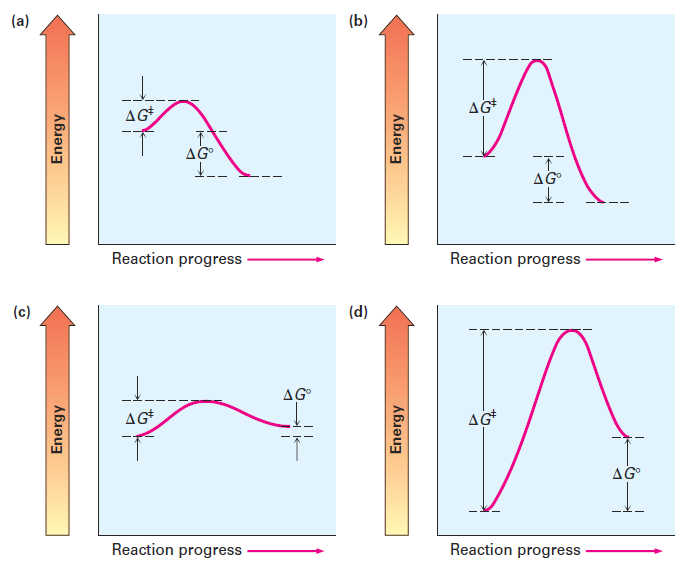
Learn About Transition State In Energy Diagram | Chegg.com An energy diagram refers to the plot which shows the reactants' relative potential energies, its transition states, as well as the products progression of the reaction with time. It is a plot between potential energy and reaction progress or time. It tells us about the reaction whether it is endothermic or exothermic. Transition states and activation energy | Open Textbooks ... A reaction starting from reactants that change into products must pass through an unstable state at the highest energy level; this is called a transition state.A certain amount of energy is required to overcome the energy barrier, and this energy, called activation energy, is represented by ΔG ‡.The magnitude of ΔG ‡ determines the rate of a chemical reaction. Transition State Theory | Introduction to Chemistry Transition state theory has been successful in calculating the standard enthalpy of activation, the standard entropy of activation, and the standard Gibbs energy of activation. Between products and reactants, there exists the transition state. The activated complex is a higher-energy, reactant-product hybrid.
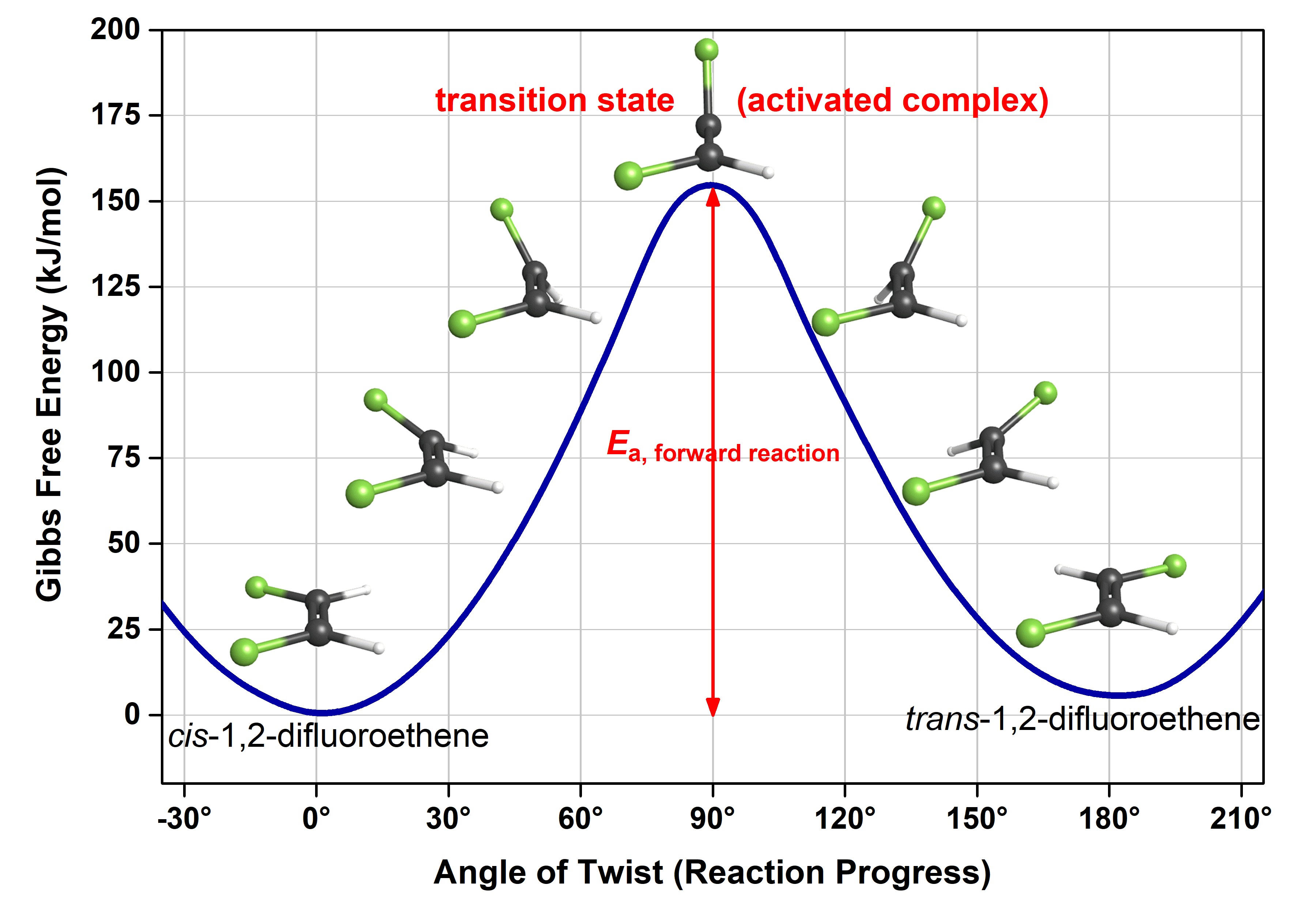
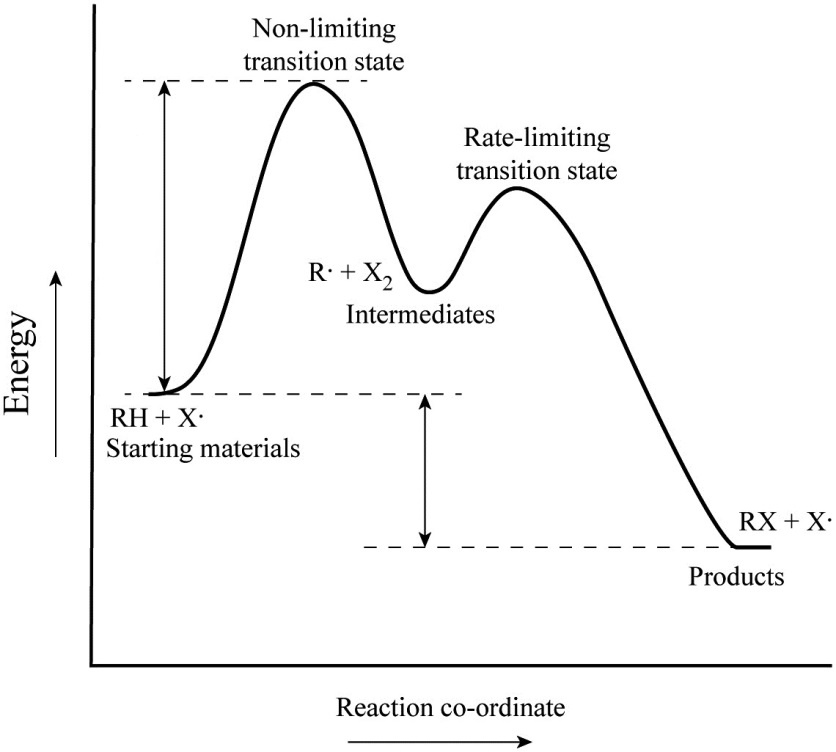
Transition state energy diagram. Energy Diagrams, Transition States, and Reactive ... Peaks on the energy diagram represent the transition states, whereas the valleys represent the reactive intermediates. As the reaction progresses, the reactants pass through an unstable state of maximum free energy, called the activated complex, or the transition state. They last for less than one picosecond and cannot be isolated. PDF Thermodynamics vs Kinetics - Columbia University transition state product Exothermic Reaction Energy Reaction Coordinate reactant transition state product Endothermic Reaction Energy Reaction Coordinate Finally, the diagram gives information about the rate of a reaction. The higher the energy of the transition state (corresponding to an increase in activation energy Ea) the slower the Energy level - Wikipedia The specific energies of these components vary with the specific energy state and the substance. Energy level diagrams. There are various types of energy level diagrams for bonds between atoms in a molecule. Examples Molecular orbital diagrams, Jablonski diagrams, and Franck–Condon diagrams. Energy level transitions Difference between intermediates and transition states A transition state cannot be isolated while an intermediate can be isolated. A transition state is a chemical species which has only fleeting existence and represents an energy maxima on reaction coordination diagram . While an intermediate lies in depression on potential energy curve .
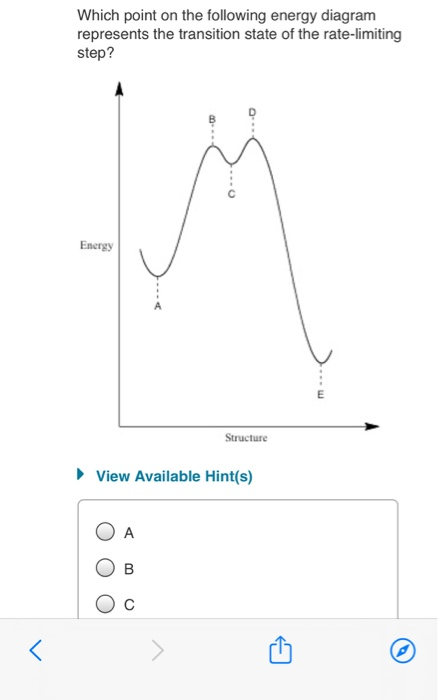
CH 368: Unit 2 - University of Texas at Austin 1. The Basic Equation. According to transition state theory, the rates of reactions are given by the following equation: where k is the rate constant for a given reaction, k is Boltzmann's constant, T is the absolute temperature, h is Planck's constant, and DG* is the free energy difference between the transition state and the reactants, i ... Solved Which point on the following energy diagram - Chegg Best Answer. This is the best answer based on feedback and ratings. Transcribed image text: Which point on the following energy diagram represents the transition state of the rate-limiting step? . Energy Structure View Available Hint (s) O O O Energy Structure View Available Hint (s) ОА Ов Ос What is the correct order of stability for the ... State Transition Diagram - an overview | ScienceDirect Topics The state transition diagram also illustrates the states and transitions of the communication protocol between the recipe phase and the equipment phase. The phase logic must adhere to the rules depicted in the state transition diagram. Only valid state transitions as depicted in Figure 8.6 may be utilized. Though the configuration of the phase ... Molecular vibration - Wikipedia A molecular vibration is a periodic motion of the atoms of a molecule relative to each other, such that the center of mass of the molecule remains unchanged. The typical vibrational frequencies range from less than 10 13 Hz to approximately 10 14 Hz, corresponding to wavenumbers of approximately 300 to 3000 cm −1 and wavelengths of approximately 30 to 3 µm.The formula for calculation of ...
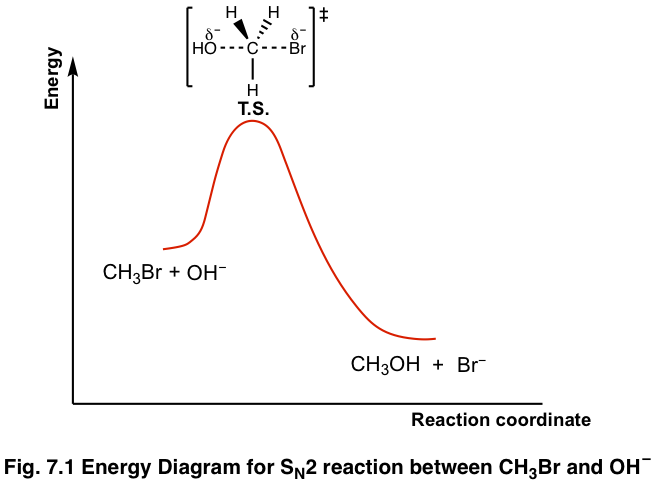
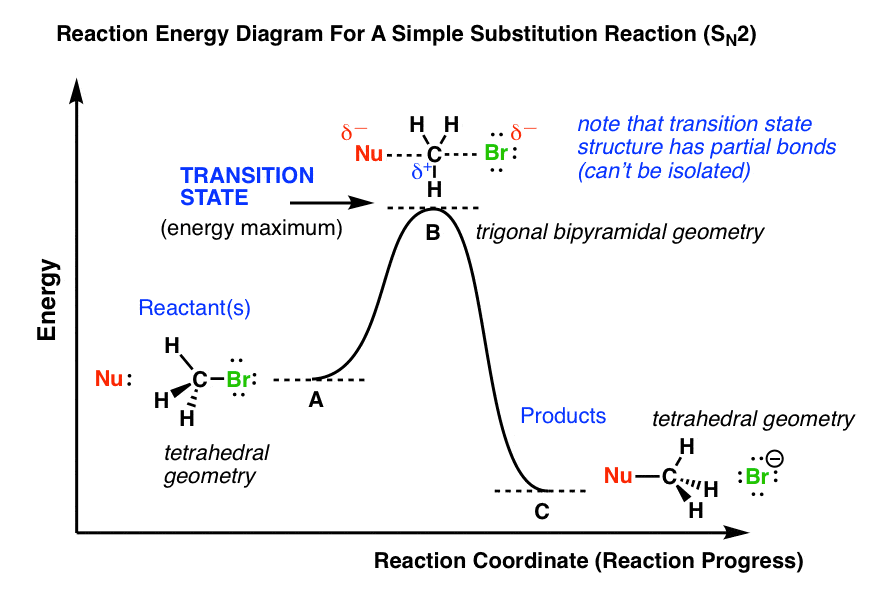
How do catalysts affect energy diagrams ... Catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy diagram in Figure 7.14), and therefore increase the reaction rate. What effect does a catalyst have on the activation energy of a reaction? The catalyst lowers the energy of the transition state for ... Energy level diagrams and the hydrogen atom The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy. 7.2 SN2 Reaction Mechanism, Energy Diagram and ... The energy changes for the above reaction can be represented in the energy diagram shown in Fig. 7.1. S N 2 is a single-step reaction, so the diagram has only one curve. The products CH 3 OH and Br - are in lower energy than the reactants CH 3 Br and OH - , indicates that the overall reaction is exothermic and the products are more stable. PDF Reactions of Alkenes - University of Texas at Austin Energy Energy Diagrams 6 • Transition state ‡: - An unstable species of maximum energy formed during the course of a reaction. - A maximum on an energy diagram. • Activation Energy, ∆G‡: The difference in Gibbs free energy between reactants and a transition state. - If ∆G‡ is large, few collisions occur with sufficient
Tsung Xu | The Clean Energy Transition, A Guide Mar 01, 2022 · But the larger impact for any energy transition, like other new technologies, is to enable us to do brand new things. Take a steam engine tinkerer in Britain in 1800, when steam engines were mostly limited to heating, improving coal mining and industrializing iron.
What is the Difference Between a Transition State and an ... Transition state is the highest point (or points) on the reaction coordinate diagram. Those are the “peaks” or the “hills” in the picture. A more strict definition is that a transition state is a molecular entity that has a lifetime no longer than a vibration that exhibits some structural characteristics of both the reactants and the ...
Energy Diagram Module Series- Part Three: Intermediates ... This is part 3 of a four part series in the Energy Diagram Module. Stay tuned for Part 4! Click on the following links to see earlier parts: Part 1. Part 2. Sometimes reactions are more complex than simply a transition state (Graph 3), which would represent a single step in the reaction mechanism.
Transition state - Wikipedia The transition state of a chemical reaction is a particular configuration along the reaction coordinate.It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger ‡ symbol.. As an example, the transition state shown below occurs during the S N 2 reaction of bromoethane with a hydroxyl anion:
PDF Energy Profile Diagrams - staff.du.edu.eg Chapter 4 2 Energy Diagram of One-Step Exothermic Reaction • The vertical axis in this graph represents the potential energy. • The transition state is the highest point on the graph, and the activation energy is the energy difference between the reactants and the transition
Transition State Theory - Concept, Formation, Formula and ... Now the diagram above shows the transition state of a chemical reaction taking place. It is basically a potential energy graph that shows the minimum energy required to convert reactants into products. From this curve, it is clear that that activation energy is a hurdle that the reactants need to overcome during the chemical reaction to get ...
Drawing the Reaction Energy Diagram of a Catalyzed ... In a typical reaction energy diagram, the reactants will be shown on the left-hand side of the diagram and the products on the right. In between the two exists a transition state that is directly ...
What is State Transition Testing? Diagram, Technique, Example In Software Engineering, State Transition Testing Technique is helpful where you need to test different system transitions. Two main ways to represent or design state transition, State transition diagram, and State transition table. In state transition diagram the states are shown in boxed texts, and the transition is represented by arrows.
Transition state theory - Wikipedia Transition state theory (TST) explains the reaction rates of elementary chemical reactions.The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.. TST is used primarily to understand qualitatively how chemical reactions take place.
A method to manipulate non-steady-state columnar-to-equiaxed ... We deduce that the transition boundary lines in , can be dilated to transition boundary areas, which enlarge the minimum deviation angle of G with respect to the six 〈001〉 dendrite tips, and therefore, are the most likely to induce the non-steady-state CET. The non-steady-state CET occurs when the G direction swings into the transition ...
Exothermic Energy Diagram: Activation Energy, Transition ... In this video, I go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. I'll ...
State-Transition Diagrams - StickyMinds State-Transition Diagrams State-transition diagrams describe all of the states that an object can have, the events under which an object changes state (transitions), the conditions that must be fulfilled before the transition will occur (guards), and the activities undertaken during the life of an object (actions).
Arrhenius Theory and Reaction Coordinates Transition State. The transition state is the high energy point between two minima along the reaction coordinate. Each step in a mechanism will have a transition state. As with the overall mechanism the rate of the reaction may only depend on the highest energy transition state as this will dominate the rate.
State Transition Table - an overview | ScienceDirect Topics Ben rewrites the state transition diagram as a state transition table (Table 3.1), which indicates, for each state and input, what the next state, S′, should be. Note that the table uses don’t care symbols (X) whenever the next state does not depend on a particular input. Also, note that Reset is omitted from the table.
PDF Teacher's Guide to "Visualizing the Transition State and ... Transition state theory (TST), also called activated complex theory, is often introduced in general chemistry courses when discussing kinetics. A reaction energy diagram is used to follow the progress of the reaction from reactants through a transition state to products (see figure 1). The reaction energy diagram plots the
Sn2 Energy Diagram - Wiring Diagrams The transition state is the point in the reaction with the highest energy level, and the difference in energy between the reagents and transition state is called the activation energy (often abbreviated as Ea). This Pin was discovered by Jessica L. Santos. Discover (and save!) your own Pins on Pinterest.The SN2 Reaction Energy Diagram ...
Transition State Theory The active intermediate is shown in transition state at the top of the energy barrier. A class of reactions that also goes through a transition state is the S N2 reaction. A. The Transition State ... The entire energy diagram for the ABC system is shown in 3-D in Figure PRS.3B-3.
Transition State Theory | Introduction to Chemistry Transition state theory has been successful in calculating the standard enthalpy of activation, the standard entropy of activation, and the standard Gibbs energy of activation. Between products and reactants, there exists the transition state. The activated complex is a higher-energy, reactant-product hybrid.
Transition states and activation energy | Open Textbooks ... A reaction starting from reactants that change into products must pass through an unstable state at the highest energy level; this is called a transition state.A certain amount of energy is required to overcome the energy barrier, and this energy, called activation energy, is represented by ΔG ‡.The magnitude of ΔG ‡ determines the rate of a chemical reaction.
Learn About Transition State In Energy Diagram | Chegg.com An energy diagram refers to the plot which shows the reactants' relative potential energies, its transition states, as well as the products progression of the reaction with time. It is a plot between potential energy and reaction progress or time. It tells us about the reaction whether it is endothermic or exothermic.




















0 Response to "39 transition state energy diagram"
Post a Comment