41 orbital diagram for f- ion
PDF Orbital diagram for zinc ion The diagram shows the number of subshell using boxes or lines for electrons (use three for p-orbitals, five for d-orbitals, and 7 for f-orbitals). In each box, the rotation of an electron is noticed using arrows, arrows up mean 1·2 spin and arrows down mean -1·2 spin. The orbital diagram for the first 18 atoms is shown below. Chlorine(Cl) electron configuration and orbital diagram Chlorine (Cl) atom electron configuration (Bohr model) K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n 2. For example, n = 1 for K orbit. The electron holding capacity of K orbit is 2n 2 = 2 × 1 2 = 2 electrons.
What Is The Electron Configuration Of F How do you write the electron configuration for F?, In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. The remaining five electrons will go in the 2p orbital. Therefore the F electron configuration will be 1s 2 2s 2 2p 5.
Orbital diagram for f- ion
Solved Construct the orbital diagram of the F- ion ... This problem has been solved! See the answer. See the answer See the answer done loading. Construct the orbital diagram of the F- ion. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (24 ratings) en.wikipedia.org › wiki › Bohr_modelBohr model - Wikipedia The Bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. This not only involves one-electron systems such as the hydrogen atom, singly ionized helium, and doubly ionized lithium, but it includes positronium and Rydberg states of any atom where one electron is far away from everything else. PDF Electron Configurations and Orbital Diagrams key 5. -3Consider the following ions: N , O -2, F -1, Na +1, Mg +2, and Al+3. a. How many electrons are present in each ion? 10 b. 2Write a single electron configuration representing all the ions. 1s 2s 2 2p 6 c. Which neutral atom possesses this electron configuration? Neon
Orbital diagram for f- ion. Fluorine (F) Orbital diagram, Electron configuration, and ... The orbital diagram for Fluorine is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The Fluorine orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the rest five electrons in 2p orbital. Orbital diagram for a ground-state electron configuration of Fluorine atom is shown below- Sodium(Na) electron configuration and orbital diagram The 2p orbital is now full. Then next an electron will enter the 3s orbital in the clockwise direction. This is clearly shown in the figure of the orbital diagram of sodium. Sodium ion(Na +) electron configuration. Ground state electron configuration of sodium(Na) is 1s 2 2s 2 2p 6 3s 1. The elements that have 1, 2, or 3 electrons in the last ... Answered: Construct the orbital diagram for the… | bartleby Construct the orbital diagram for the ion Ca2+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Fill all group 1 targets. Not all group 2 targets will be filled. 1s 2s 2p 3s 3p 3d 4s 4p 4d 1L 1L 1L 1L 1L 1L 1L 11 1L 1s 2s 2p 3s 3p Construct the orbital diagram of the f ion - Soetrust Construct the orbital diagram of the f ion. By soetrust March 29, 2022. Gamers!!! Amazon Luna launches with freebies for Prime subscribers. Amazon Luna special offer for Prime members!
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine Molecular orbital diagram of hydroxide ion? - Chemistry ... Bookmark this question. Show activity on this post. I would like to understand how to create a molecular orbital diagram for the hydroxide ion from scratch. This includes understanding the shape of the molecular orbital. Here is an attempt I have come up with: Where the top MO is sigma* and the bottom is simply sigma. But this makes no sense. Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... Orbital Diagram For Fe3+ Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms.
Calcium Orbital Filling Diagram - schematron.org For example, the 5s orbital is of lower energy than the 4d orbital (see Figure 3 for a complete pattern of orbital levels). Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The orbital filling diagram of lithium. The electron configuration of lithium is 1s²2s¹. Construct the orbital diagram of the F^- ion. | Study.com Orbital Diagram: Orbital diagram shows how electrons are distributed in various kinds of shells in the increasing order for a particular ion or element. The filling of electrons is studied and ... Nitrogen Orbital diagram, Electron configuration, and ... Orbital diagram:-A orbital diagram is simply a pictorial representation of the arrangement of electrons in the orbital of an atom, it shows the electrons in the form of arrows, also, indicates the spin of electrons.Electron configuration:- Electron configuration is the arrangement of electrons in atomic orbitals.It shows the electrons in numbers, It doesn't show the details on the spin of ... 39 construct the orbital diagram of the f ion - Diagram ... Construct the orbital diagram of the f ion. How to Do Orbital Diagrams - Sciencing The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital.
Enter the orbital diagram for the ion au+. An orbital diagram is the pictorial representation of shells in an atom by using square boxes (one box for s-orbital, three boxes for p-orbitals, five boxes for d-orbitals and seven boxes for f-orbitals) those boxes are filled by electrons using the following principles: Aufbau principle, Hund's rule, and Pauli's exclusion principle. Fundamentals
Molecular Orbital (MO) Diagram for F2(2+) - YouTube When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ...
en.wikipedia.org › wiki › MoleculeMolecule - Wikipedia The simplest of molecules is the hydrogen molecule-ion, H 2 +, and the simplest of all the chemical bonds is the one-electron bond. H 2 + is composed of two positively charged protons and one negatively charged electron, which means that the Schrödinger equation for the system can be solved more easily due to the lack of electron–electron ...
Draw molecular orbital diagram for ${{F}_{2}}$ molecule ... 101.4k + views. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic configuration. Thus, the magnetic property can be explained when we know electronic configuration of molecules.
Spacecraft propulsion - Wikipedia Spacecraft propulsion is any method used to accelerate spacecraft and artificial satellites. In-space propulsion exclusively deals with propulsion systems used in the vacuum of space and should not be confused with space launch or atmospheric entry.. Several methods of pragmatic spacecraft propulsion have been developed each having its own drawbacks and advantages.
Solved Construct the orbital diagram of the F^-ion ... Construct the orbital diagram of the F^-ion. Question: Construct the orbital diagram of the F^-ion. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and ...
How To Draw Molecular Orbital Diagram Of Co - Drawing ... Electronic configuration of co molecule is: Draw the orbital diagram for the ion co2+. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The bonding mos are the 2σ, 1πx, 1πy, and 3σ, which gives 2 +2 +2 +2 = 8 bonding electrons. The content is presented using short focussed and interactive screencast.
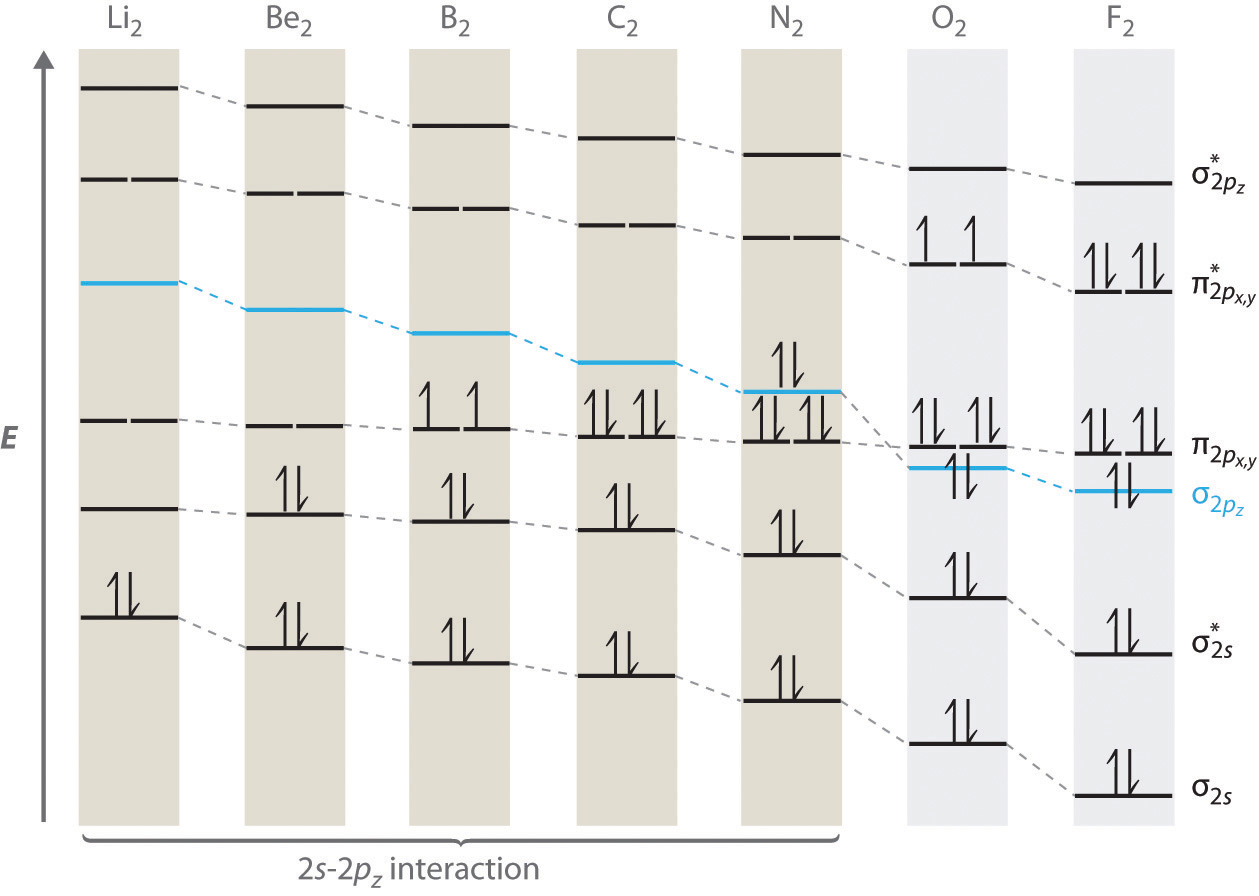
40 draw the molecular orbital (mo) electron diagram for ... At the moment I'm learning about molecular orbital diagrams for homonuclear molecules, namely: B2, C2, N2, O2, F2, and Ne2. I understand that the energy of the 2p sigma bond is at a higher level for B2, C2, and N2, leading to the 2p sigma bond and the 2p pi bond switching places in the MO diagram (with 2p pi bond appearing under 2p sigma bond) for B, C, and N but not for O, F, or Ne.
Orbital Diagram For Au+ - schematron.org Click within the orbital to add electrons. Jul 21, · Write orbital diagram for Au+ Determine if the ion is diamagnetic or paramagnetic. please helpStatus: Resolved.what is the orbital diagram for Au+, how do you fit the f orbitals in?what is the orbital diagram for Au , how do you fit the f orbitals in?
What is the electron configuration of F^-? | Socratic F: 1s22s22p5 Now, the F− anion is formed when 1 electron is added to a neutral fluorine atom. Notice that the 2p-subshell of the neutral atom contains 5 electrons. Its maximum capacity is actually 6 electrons, two electrons for each p-orbital. This means that the extra electron will be added to one of the three 2p-orbitals, let's say to 2py.
Construct the orbital diagram of the F- ion. If you can't find your institution, please check your spelling and do not use abbreviations. If your institution is not listed, please visit our Digital Product Support Community .
acidity - employees.csbsju.edu The diagram for BH3F- ion is really a superposition of the two diagrams before, except that the HOMO and LUMO have formed a new bonding and antibonding orbital for the new B-F bond. Figure AB4b.6. Molecular orbital interaction diagram for formation of an adduct between a fluoride ion and borane. Problem AB4b.1.
Electron Configuration for Fluorine (F) - TerpConnect In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...24 Oct 2016 · Uploaded by Wayne Breslyn
Fluorine(F) electron configuration and orbital diagram Fluorine (F) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
Molecular Orbital Theory - Chemistry Ion Predictions with MO Diagrams Give the molecular orbital configuration for the valence electrons in \({\text{C}}_{2}{}^{\text{2−}}.\) Will this ion be stable? Solution Looking at the appropriate MO diagram, we see that the π orbitals are lower in energy than the σ p orbital.
Construct the orbital diagram of the f ion The Fluoride ion formed by addition of electron to its neutral state. F + e^- rightarrow F^- Thus, F^- ion has 10 electrons. The electronic configuration of Fluoride ion is 1s^2 3s^2 2p^6. As the energy of the atomic orbital is 1s^2 < 2p^6 (2p^2_x = 2p^2_y = 2p^2_z), the orbital energy diagram is represented as shown below:
PDF Electron Configurations and Orbital Diagrams key 5. -3Consider the following ions: N , O -2, F -1, Na +1, Mg +2, and Al+3. a. How many electrons are present in each ion? 10 b. 2Write a single electron configuration representing all the ions. 1s 2s 2 2p 6 c. Which neutral atom possesses this electron configuration? Neon
en.wikipedia.org › wiki › Bohr_modelBohr model - Wikipedia The Bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. This not only involves one-electron systems such as the hydrogen atom, singly ionized helium, and doubly ionized lithium, but it includes positronium and Rydberg states of any atom where one electron is far away from everything else.
Solved Construct the orbital diagram of the F- ion ... This problem has been solved! See the answer. See the answer See the answer done loading. Construct the orbital diagram of the F- ion. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (24 ratings)

0 Response to "41 orbital diagram for f- ion"
Post a Comment