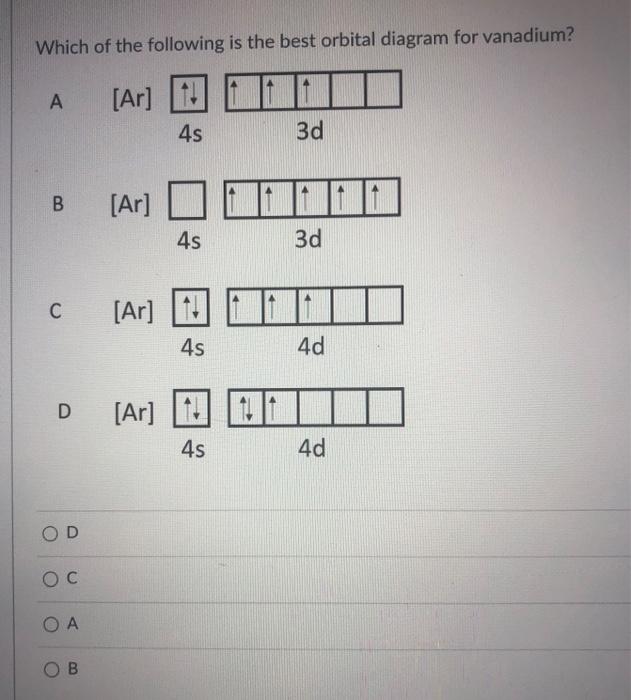
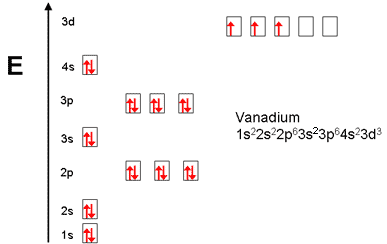
40 choose the correct orbital diagram for vanadium.
how many valence electrons does vanadium have The free energy of an electronâ hole pair is smaller than the band gap energy due to the translational entropy of the electrons and holes â ¦ The chemical symbol for Vanadium is V. Vanadium is a hard, silvery grey, ductile, and malleable transition metal. 1: Bohr diagrams: Bohr diagrams indicate how many electrons fill each principal shell. Chapter 9 electrons in atoms and the periodic table 100% ... 37) The orbital diagram for fluorine shows 1 unpaired electron in a p orbital. 38) The correct electron configuration for magnesium is: 1s 2 2s 2 2p 6 3s 3. 39) The element manganese (symbol = Mn) has five valence electrons. 40) Bromine has 17 valence electrons. 41) Bromine has 28 core electrons.
Electron configurations of the elements (data page ... Main article: Electron configuration. This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Electron configurations of elements beyond hassium (element 108 ...

Choose the correct orbital diagram for vanadium.
Lewis Structures: Learn How to Draw Lewis Structures ... Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16. Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element. 42 orbital diagram of titanium As illustrated in the following diagram, the electron pair of the pi-orbital may be shared with an empty d-orbital to form a sigma-like bond (light blue arrow). This leaves the double bond function electron deficient, a condition that may be remedied by the sharing of a d-electron pair with the empty antibonding-pi-orbital (a pi-like backbonding). Electron Configuration Worksheet 3 Answer Key - Islero ... CH301 Fall 2010 Worksheet 3 Answer Key. Displaying all worksheets related to electric configuration. Pin By Recursos Escolares On Chemistry Chemistry Classroom Chemistry Lessons Chemistry Basics Electron configuration review worksheet answer key.Electron configuration worksheet 3 answer key. 3 4 covalent bonds and lewis structures in 1916 g. A letter indicates the type of orbital.
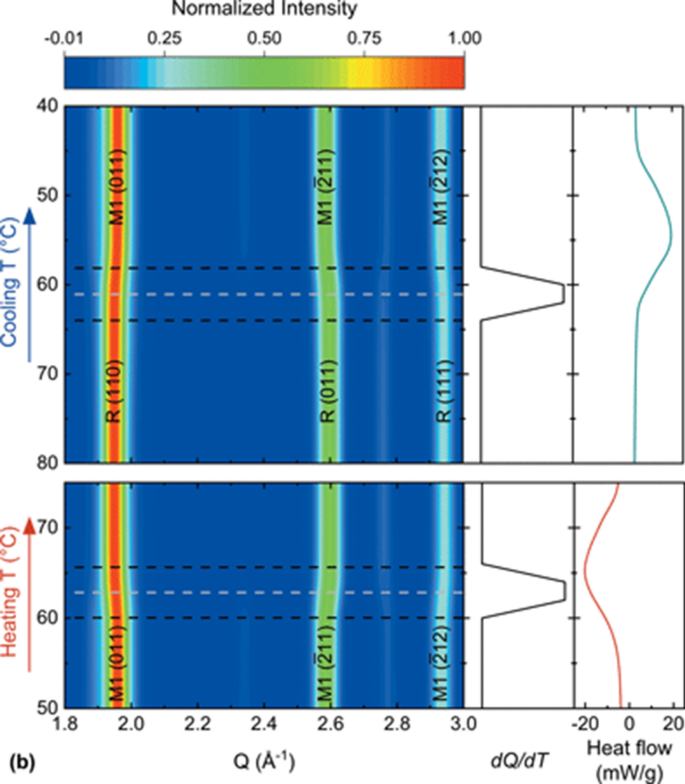
Choose the correct orbital diagram for vanadium.. The metal-insulator phase change in vanadium dioxide and ... Left: Schematic diagram of vanadium site dimerization for M 1 and M 2 phases and its absence in the rutile phase, with doubling the primitive rutile cell along with Oz in M 1 and M 2. The dashed green lines show the lattices of M 1 and M 2. M 2 has a magnetic moment of one Bohr magneton in each unpaired V. Ultrafast and long-time excited state kinetics of an NIR ... Based on the natural orbital occupation numbers, these states feature the (t 2g) 2 configuration at the vanadium center and the π → π* excitation at the ddpd ligand. In order to clarify the character of the states T 10 -T 15 , we use charge transfer numbers 63 and partition V III Cl 3 (ddpd) into three fragments ( Fig. 3(b) ): the metal ... 41 electron configuration box diagram - diagram Download this The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +½). Effect of the projector augmented wave potentials on the ... Taking the melting simulation of vanadium as an example, it is much cheaper to choose the PAW potential containing only five valence electrons (labeled V) than the version containing 13 valence electrons (labeled V_sv). Thus, the PAW potential V should also be tested as a candidate.
Orbital Diagram For Bromine, Atomic Numbers Electron ... Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. So you have the carbon two S orbital, and you have the carbon two P orbitals. Stack The Sub Shells In Order Of Energy, With The Lowest-energy Sub Shell At The Bottom And The Highest-energy Sub Shell At The Top. P-Block Elements on the Periodic Table ... - Study.com Elements whose outermost electrons are in the p-orbital are known as 'P-Block' elements, found in a block on the periodic table. Explore an overview of the properties of this array of 35 elements ... Element 4s2 [7ULN0Z] Problem 4SAQ: Choose the correct orbital diagram for vanadium. Krypton [Ar] 3d10 4s2 4p6. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 5s2 4d10. First of all, I hate when this happens! Your teacher is actually wrong. 98154 13 3 2740 933. Now we have 2 more electrons to place into orbitals. Thus in the building-up process for the lanthanoids. Qualitative tests on vo acac 2 pdf - Australian Manuals ... Qualitative molecular orbital diagram of a d 0 metal-oxo fragment (empty metal d orbitals in an octahedral field on left, full oxygen p orbitals on right). Here it can be seen that d 1-2 electrons fill a nonbonding orbital and electrons d 3-6 fill anti-bonding orbitals, which destabilize the complex.
Electron Configuration Chart of All Elements (Full Chart) Electron Configuration Chart of All Elements (Full Chart) November 1, 2021 March 7, 2021 by Admin. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no. Chapter 9 electrons in atoms and the periodic table 100% ... Chapter 9 Electrons in Atoms and the Periodic Table 9.1 True/False Questions 1) When the elements are arranged in order of increasing number of protons, certain sets of properties recur periodically. 2) The early scientists who developed the quantum-mechanical model were bewildered by the model and it altered our fundamental view of matter. 3) Light is a type of […] Chapter 9 electrons in atoms and the periodic table 100% ... Chapter 9 Electrons in Atoms and the Periodic Table. 9.1 True/False Questions. 1) When the elements are arranged in order of increasing number of protons, certain sets of properties recur periodically. 2) The early scientists who developed the quantum-mechanical model were bewildered by the model and it altered our fundamental view of matter. 40 choose the valence orbital diagram that represents the ... CH. 3 Hw b Flashcards - Quizlet Choose the valence orbital diagram that represents the ground state of Zn. 4s:^>(4s^2) 3d:^> ^> ^> ^> ^> (3d^10) The element that corresponds to the electron configuration 1s^2 2s^2 2p^6 3s^2 3p^6 4s^1 3d^5 is _____. iron titanium Week 5 - Recitation (1st for test 2) - GitHub Pages An orbital that penetrates into ...
Electron Configuration Worksheet 3 Answer Key » Semanario ... Electron configurations worksheet answer key. In this video youll practice 3. Fill in the electron configurations for the elements given in the table. 3 4 covalent bonds and lewis structures in 1916 g. An electron configuration is a method of indicating the arrangement of electrons about a nucleus.
4s2 Element [G647SX] The element that follows a half-filled subshell must now put an electron in a previously occupied orbital, creating stronger, intraorbital electron repulsion. Now we have 2 more electrons to place into orbitals. Định nghĩa Ngày nay noble gas Any of the elements found in Group 8 at the far right of the Periodic Table.
Ground State Electron Configuration ... - Study.com Fully filled or half filled d orbitals are stable, so, some rearranging is necessary if a d orbital has 4 or 9 electrons. An electron from the s orbital will transfer to the d orbital. A good ...
Titanium oxide and chemical inhomogeneity in the ... Row 4: K p -V sys diagram showing the expected and observed orbital and radial velocities. In the case of different velocities, the observed velocity is indicated with an opaque line.
4s2 Element [UEKJNV] Problem 4SAQ: Choose the correct orbital diagram for vanadium. These periodic tables contain each element's electron configuration along with the atomic number, element symbol, element name, and atomic mass.
Electron Configuration Worksheet 3 Answer Key → Waltery ... Electron Configurations and Periodic Trends 1. Rt has three extra electrons 11 02 2 12. Orbital Diagrams Doc Chemistry Classroom Teaching Chemistry Electron Configuration Just fancy it by voting.Electron configuration worksheet 3 answer key. The electron configurations in this worksheet assume that lanthanum la is the first element in the 4f block and that actinium […]
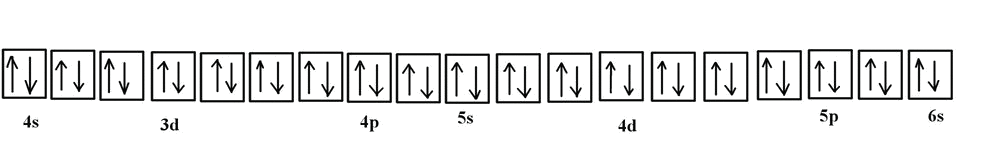
Orbital Diagram of All Elements (Diagrams given Inside) Orbital Diagram of All Elements (Diagrams given Below) January 1, 2022 April 10, 2021 by Admin Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below.
kr 5s2 4d10 electron configuration More specifically, this element will have its highest-energy electrons located in a 5p-orbital. [Kr]5s2 [Kr]4d2 [Kr]5s24d2 [Ar]4s23d104p6 [Kr]5s24d4 2) Choose the electron configuration. That's called a noble gas. (4p3) (5p2) What element is Xe . .
Electron Configuration Worksheet 3 Answer Key ... Ppt rar and also zip data. Or pull onto electrons is known as electronegativity this can be related to electron configuration worksheet 2 answer key we are living in an era of. Lewis Dot Structure Mini Lesson And Worksheet Chemistry Worksheets Chemistry Classroom Mini Lessons Selenium 1s2 2s2 2p 3s2 3p6 4s2 3d10 4p4.Electron configuration […]
Nickel Configuration Full Electron [CLFD2K] Given two configurations, the atom would "choose" the more stable one After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d9 21 Plan: Assume that the electron is in the ground state configuration and that electrons fill in a px-py-pz order To write a complete electron configuration for an uncharged atom, Determine the number of electrons in the atom from its ...
Electron Configuration Worksheet 3 Answer Key | GBGYABA ... By choosing this electron configuration worksheet and lots more answer key you get amazing results the bhj electron configuration worksheet and lots more answer key the simplest way of defining a worksheet is that it is a mono spreadsheet that is provide. Use the orbital filling diagrams to complete the table.
Electron Configuration Worksheet 3 Answer Key - Islero ... CH301 Fall 2010 Worksheet 3 Answer Key. Displaying all worksheets related to electric configuration. Pin By Recursos Escolares On Chemistry Chemistry Classroom Chemistry Lessons Chemistry Basics Electron configuration review worksheet answer key.Electron configuration worksheet 3 answer key. 3 4 covalent bonds and lewis structures in 1916 g. A letter indicates the type of orbital.
42 orbital diagram of titanium As illustrated in the following diagram, the electron pair of the pi-orbital may be shared with an empty d-orbital to form a sigma-like bond (light blue arrow). This leaves the double bond function electron deficient, a condition that may be remedied by the sharing of a d-electron pair with the empty antibonding-pi-orbital (a pi-like backbonding).
Lewis Structures: Learn How to Draw Lewis Structures ... Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16. Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element.
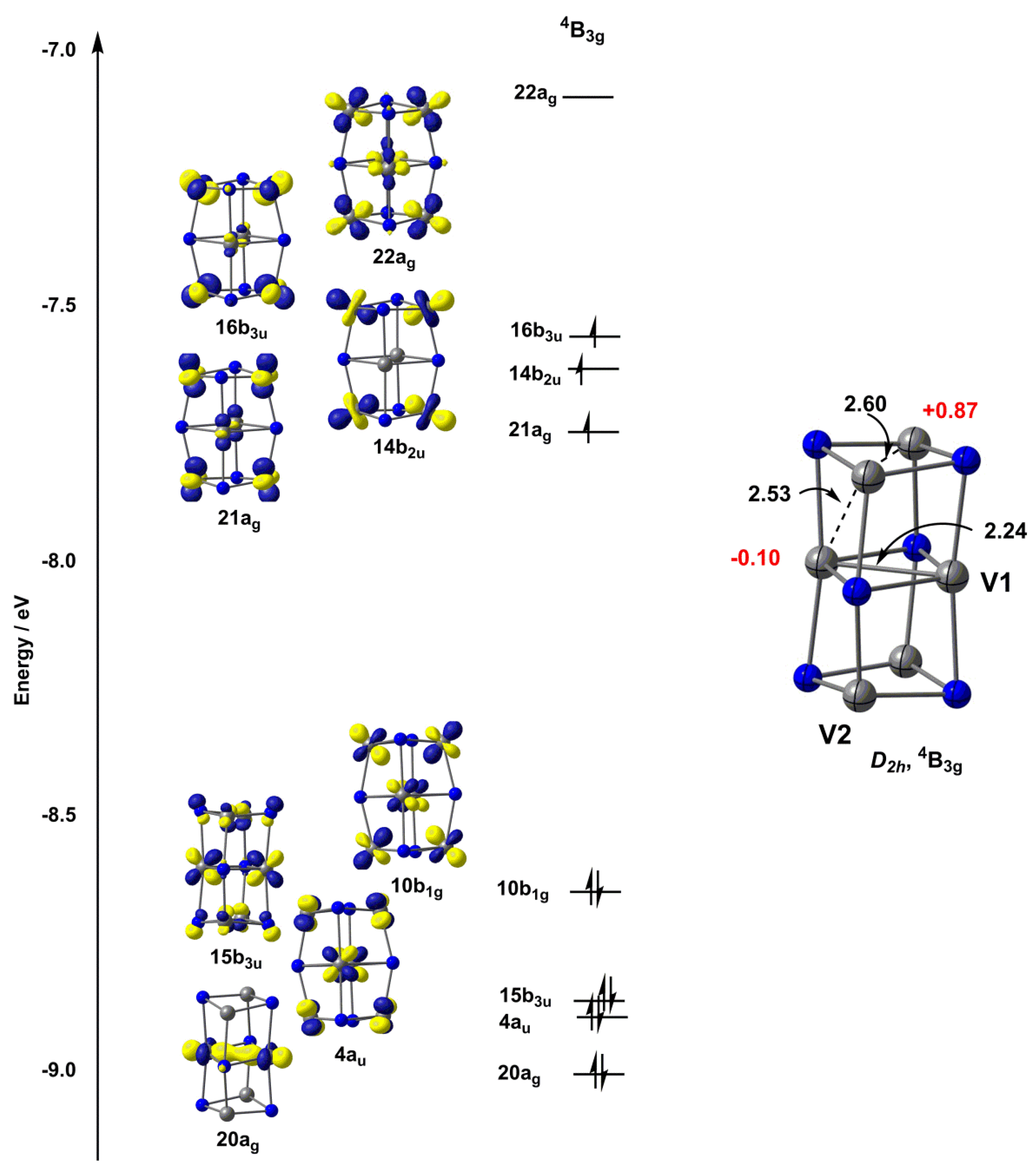




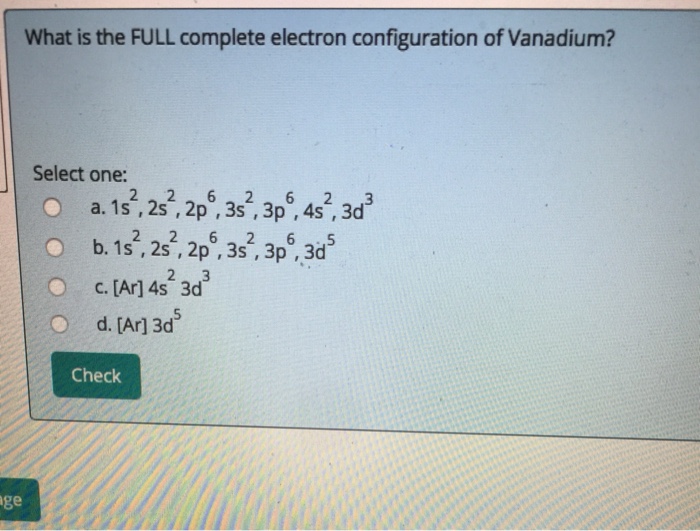




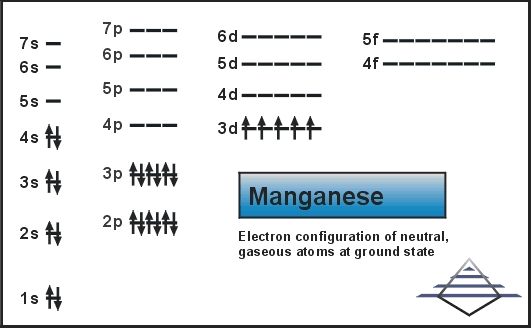


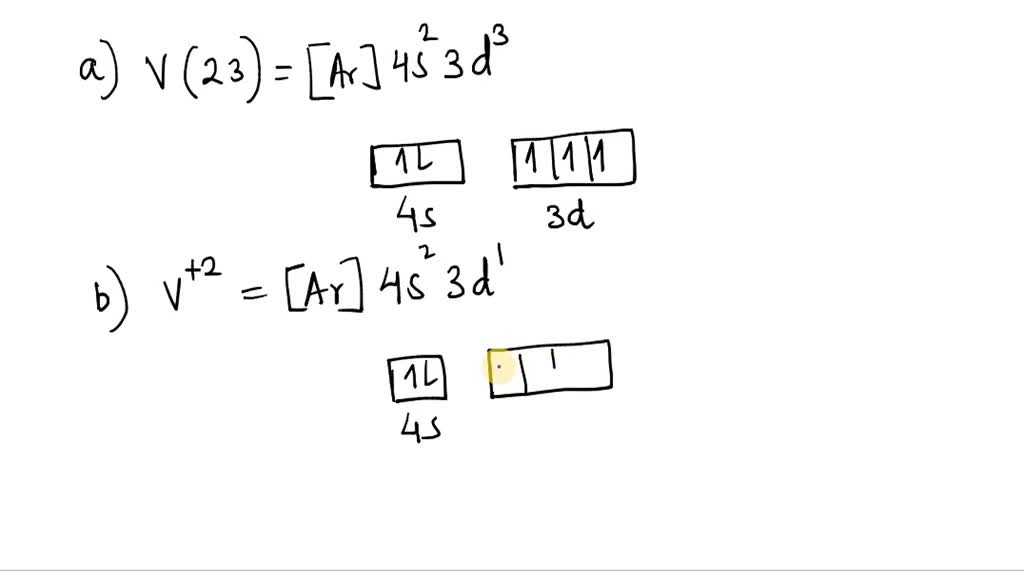





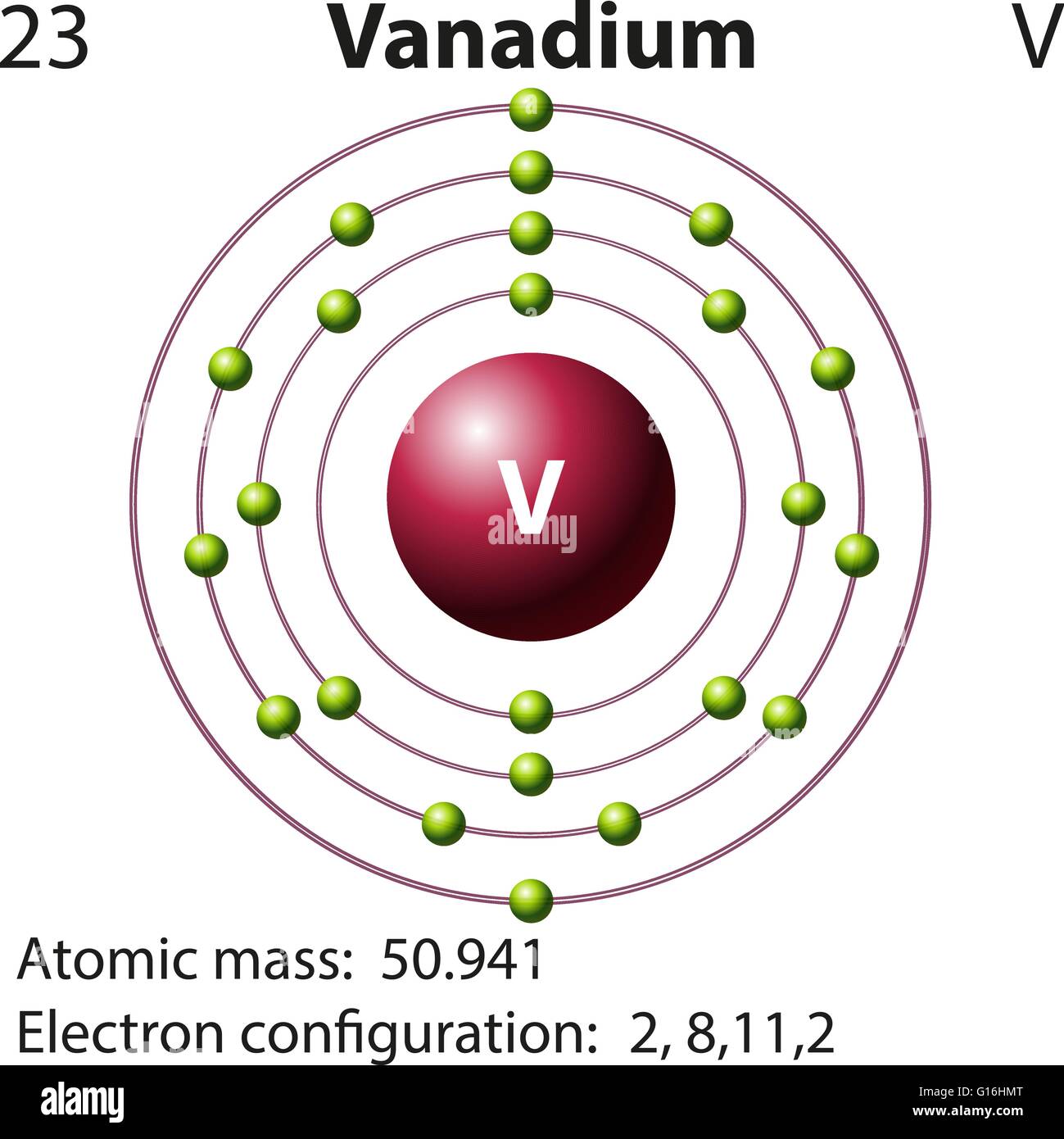
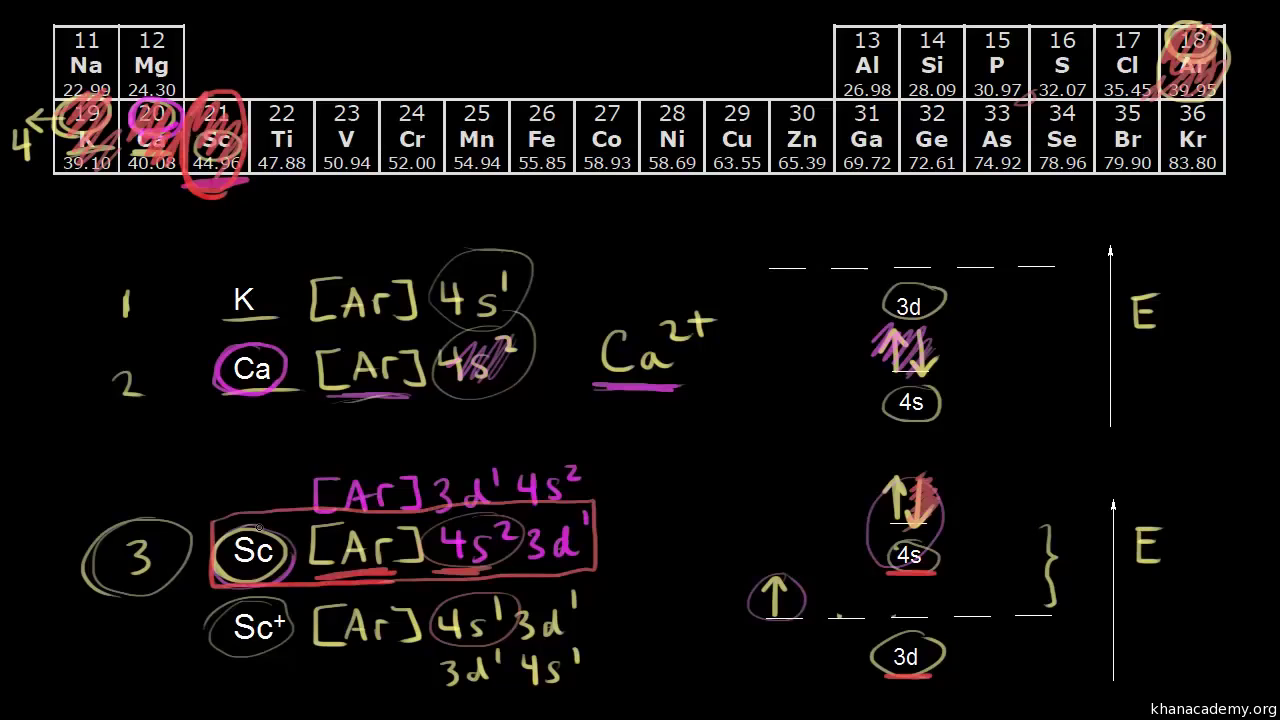


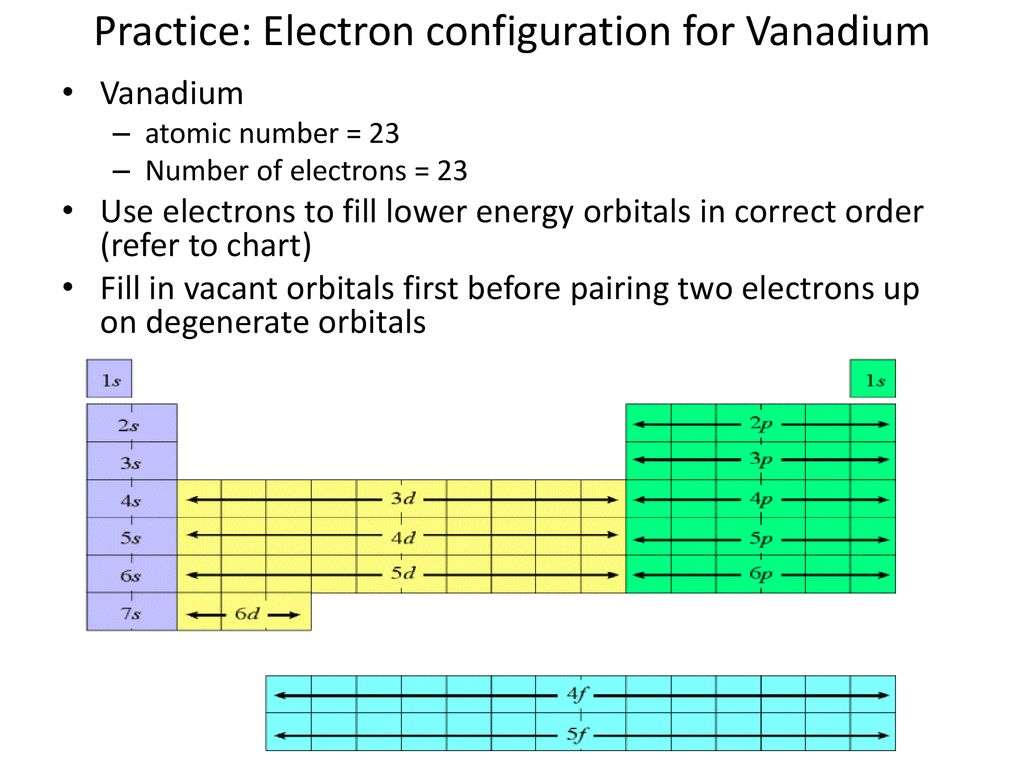

0 Response to "40 choose the correct orbital diagram for vanadium."
Post a Comment