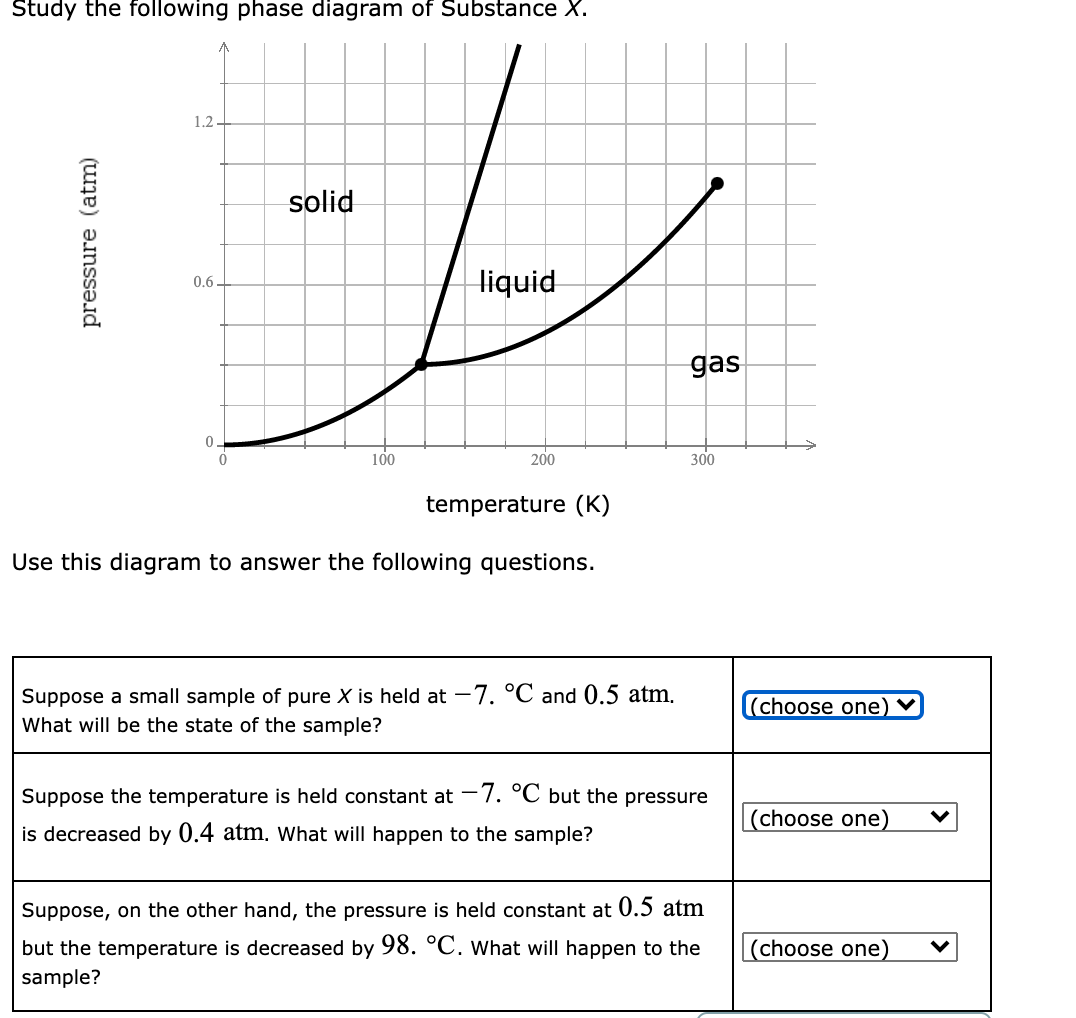
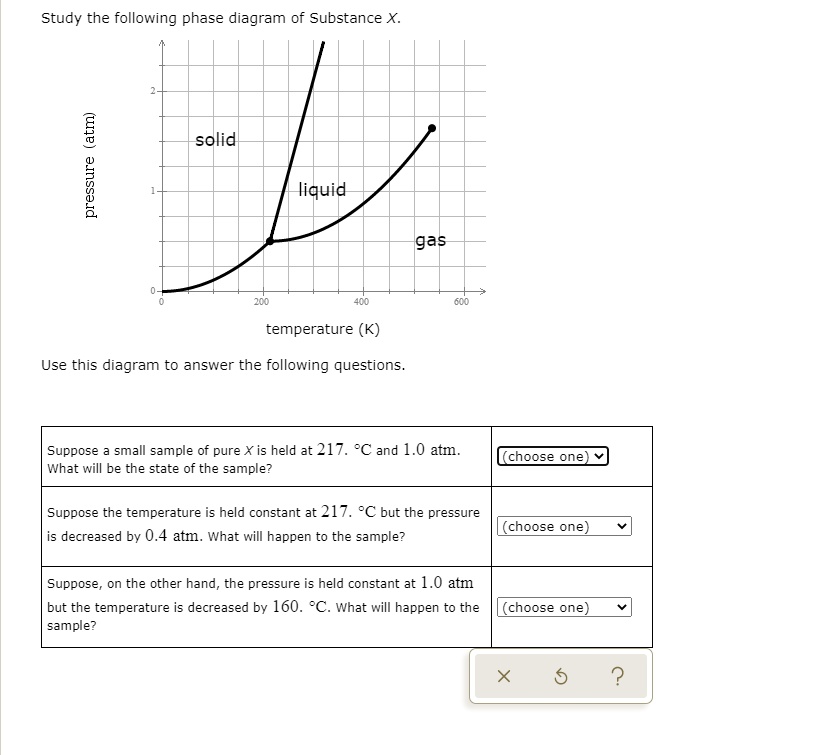
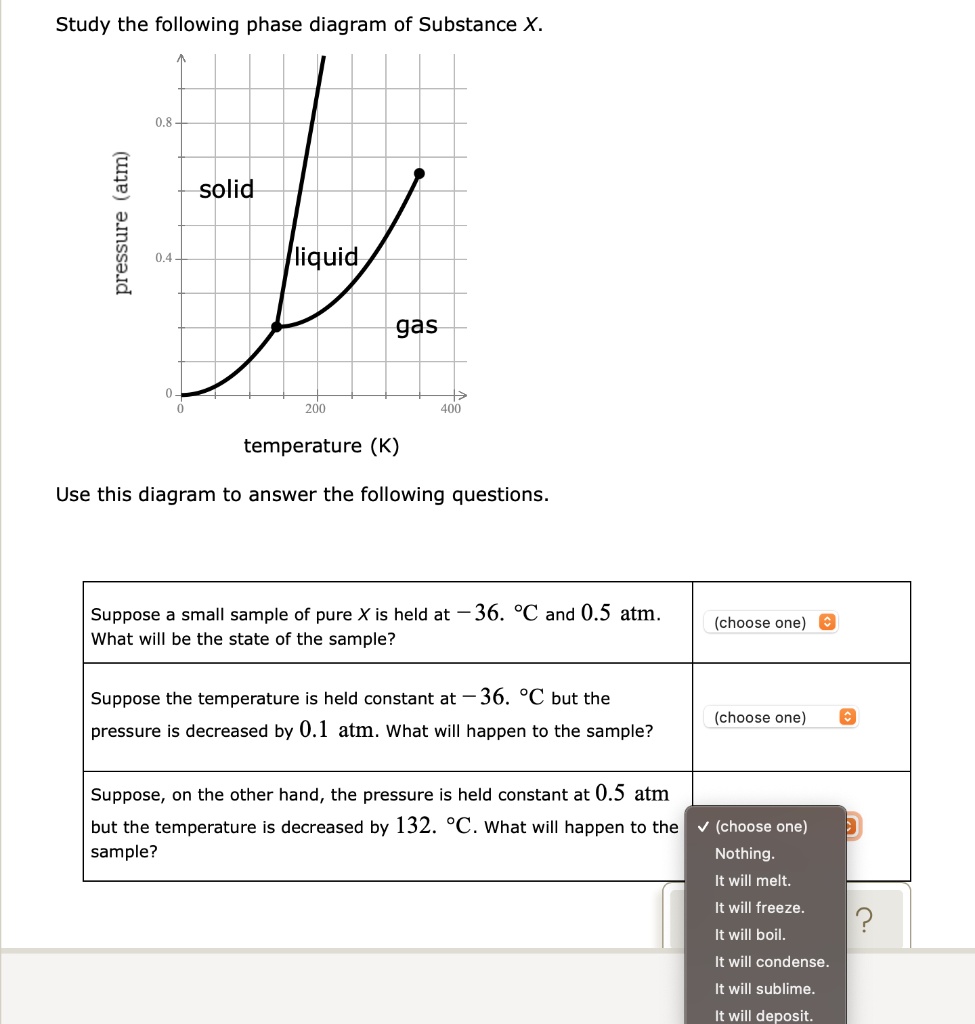
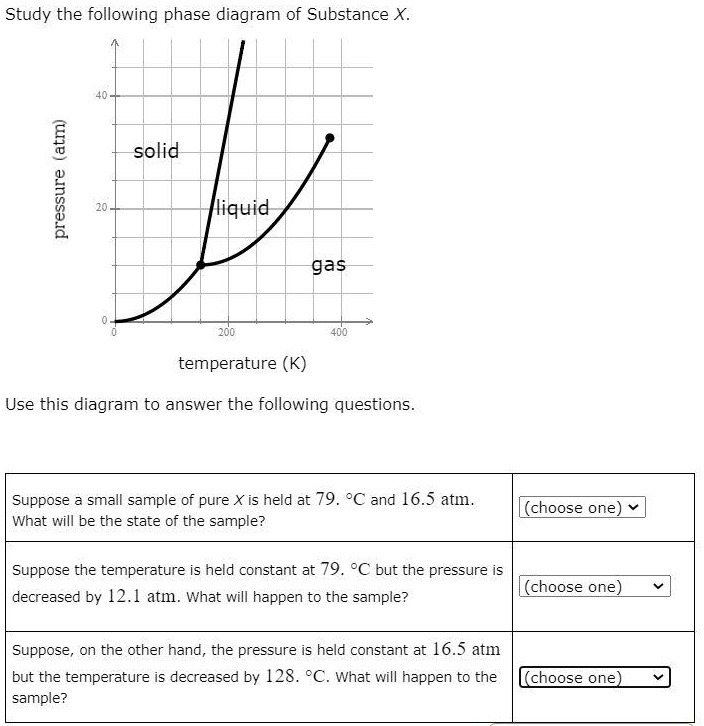
39 study the following phase diagram of substance x.
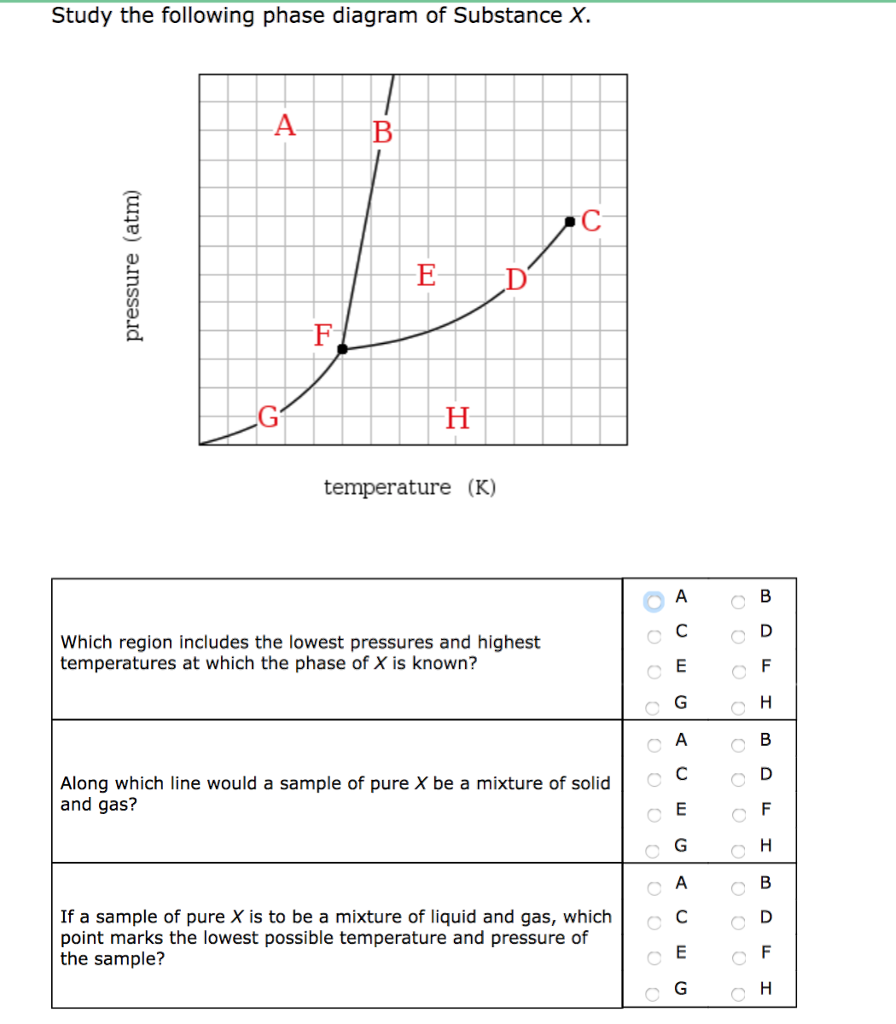
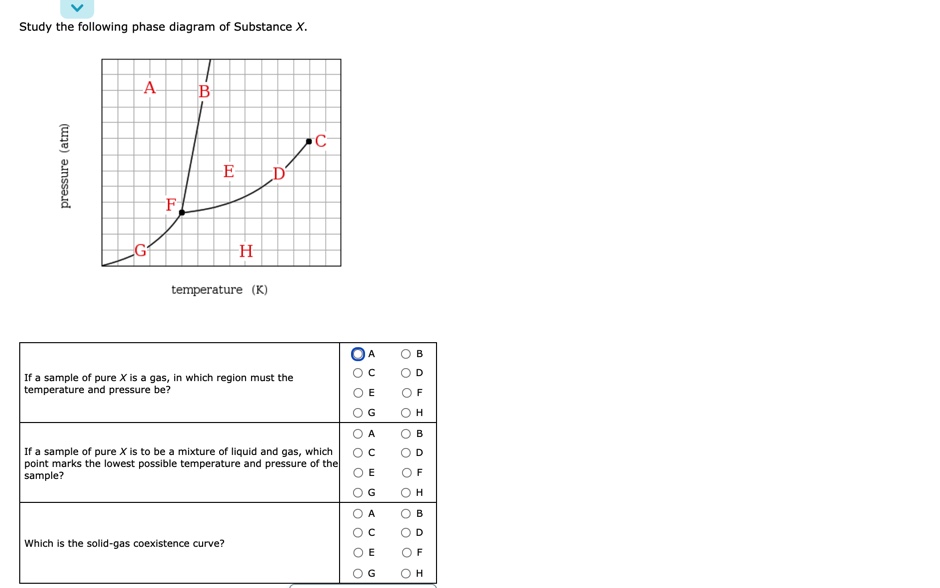
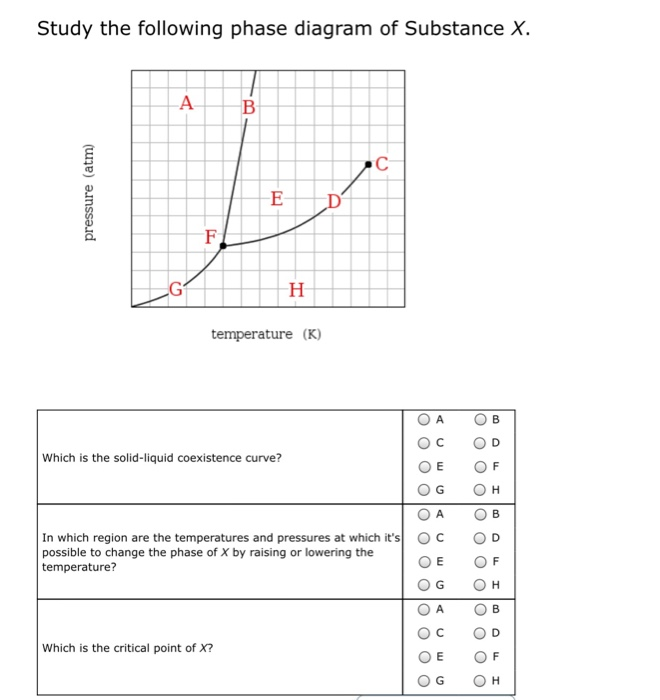
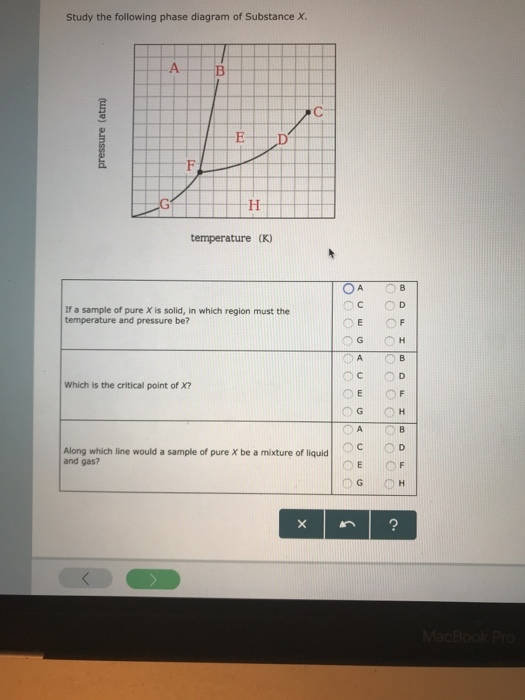
Solved Study the following phase diagram of Substance X ... Study the following phase diagram of Substance X. .40.802004006000 solid liquid gas temperature(K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at 2.°C and 0.4atm. What will be the state of the sample? Suppose the temperature is held constant at 2.°C but the pressure is decreased by 0.1atm. Answered: Study the following phase diagram of… | bartleby Study the following phase diagram of Substance X. A C E D' F, G temperature (K) A In what region would a sample of pure X be a gas? E O F G A ов O D Along which line would a sample of pure X be a mixture of liquid and gas? E O F он A O B O D Which is the triple point of X? E O F он B. O O O Ol o O O O olo o o olo o O pressure (atm)
Solved Study the following phase diagram of Substance X. A ... Study the following phase diagram of Substance X. 12 solid pressure (atmi liquid gas 100 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -84.
Study the following phase diagram of substance x.
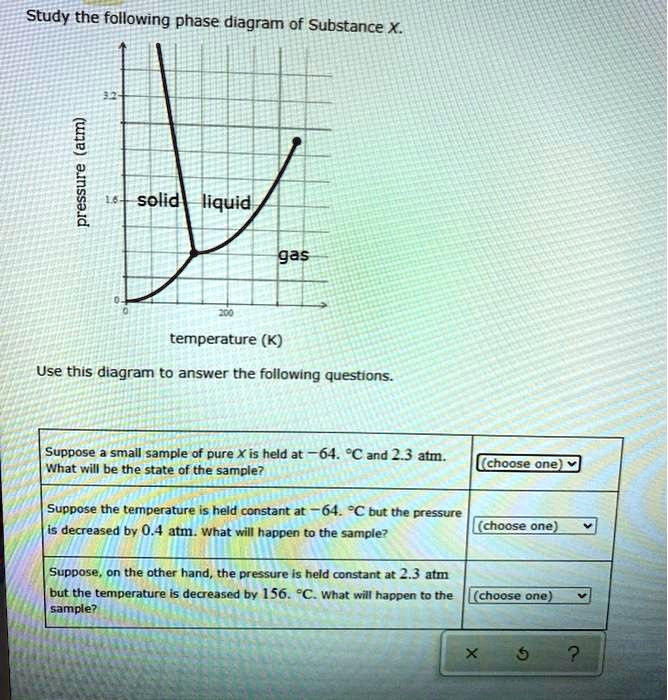
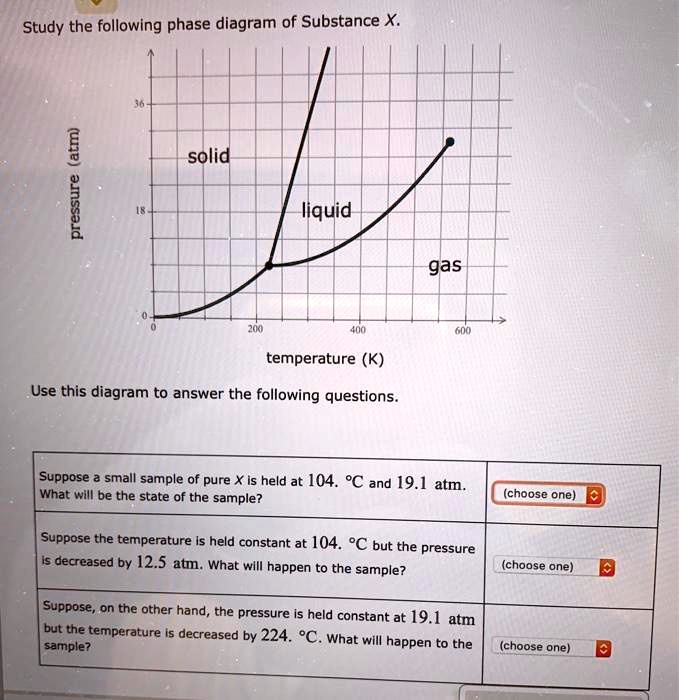
Study the following phase diagram of Subs... - Physical ... Study the following phase diagram of Substance X. 3.2- solid pressure (atm) 16 liquid gas 100 temperature (K) Use this diagram to answer the following questions Suppose a small sample of pure X is held What will be the state of the sample? 43. °C and 2.4 atm. choose one) e Suppose the temperature is held constant at 43. °C but the pressure is ... Answered: Study the following phase diagram of… | bartleby Science Chemistry Q&A Library Study the following phase diagram of Substance X. 16- solid liquid gas 100 200 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at What will be the state of the sample? 104. °C and 6.1 atm. (choose one) v (choose one) Suppose the temperature is held constant at - 104. °C but the pressure is increased by ... Solved Study the following phase diagram of Substance X ... 100% (14 ratings) Transcribed image text: Study the following phase diagram of Substance X. pressure (atm) ED temperature (K) Ο Which is the triple point of X? η Ι Φ Ο Which line must the temperature and pressure have crossed if a solid sample of X is observed to melt? OOOOOOOOOOOO η OoooOOOOOO Ι Φ Ο If a sample of pure X is a gas, in ...
Study the following phase diagram of substance x.. Study the following phase diagram of Substance X. solid ... Transcribed Image Text: Study the following phase diagram of Substance X. 24- solid liquid 1.2- gas 0. 200 400 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at 56. °C and 1.0 atm. (choose one) v What will be the state of the sample? Suppose the temperature is held constant at 56. °C but the pressure is |(choose one) decreased by ... [Solved] V Study.' the following phase diagram of ... V Study.»' the following phase diagram of Substance X. pressure [atm] temperature {K} Use this diagram to answer the following questions. Suppose a small sample of pure X is held at —T3. "C and 15-1 aim. What will be the state of the sample? Suppose the temperature Is held constant at —T3. DC but the pressure 1? Answered: Study the following phase diagram of… | bartleby Study the following phase diagram of Substance X. A В E D' temperature (K) A If a sample of pure X is to be a mixture of liquid and gas, which point marks the lowest possible temperature and pressure of the sample? Study the following phase diagram of Subs... - Physical ... Study the following phase diagram of Substance X. 3.2- solid pressure (atm) 16 liquid gas 100 temperature (K) Use this diagram t 43. °C and 2.4 atm. choose one) e Suppose the temperature is held constant at 43. °C but the pressure is decreased by 2 atm.
[Solved] i v 1 Study the fdlldwlng phase diagram of ... i v 1 Study the fdlldwlng phase diagram of Substance X. 11 I z . _ n. -_;__J_. _3 I] 100 2m am pm [arm] , temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X ls held at "* 115. "C and 17.3 31131. what will: be the state a! the sample? Suppose the temperature Is held constant at - "5. please help Study the following phase diagram of Substance ... Study the following phase diagram of Substance X. 2.4 pressure (atm) 1.2 solid liquid gas 200 400 600 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -9. °C and 1.1 atm. 38 study the following phase diagram of substance x ... From the phase diagram above, the minimum pressure at which this substance can exist in the liquid phase is 0.45 atm Answered: Study the following phase diagram of… | bartleby Study the following phase diagram of Substance X. 2.8 solid liquid 1.4 gas 400 600 temperature (K) Use this diagram to answer the following questions. Solved Study the following phase diagram of Substance X ... Transcribed image text: Study the following phase diagram of Substance X. solid liquid gas 100 200 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at-79. °C and 1.4 atm. What will be the state of the sample? (choose one) Suppose the temperature is held constant at-79. °C but the pressure is decreased by 0.4 atm.
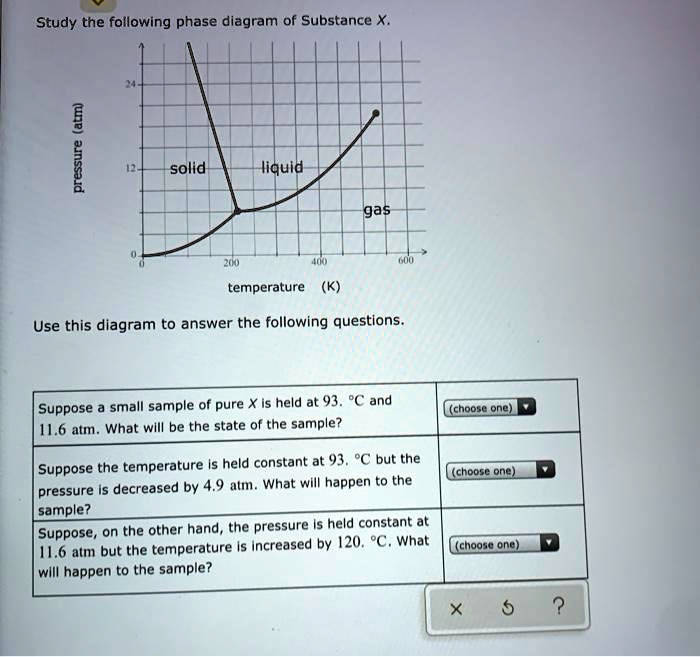
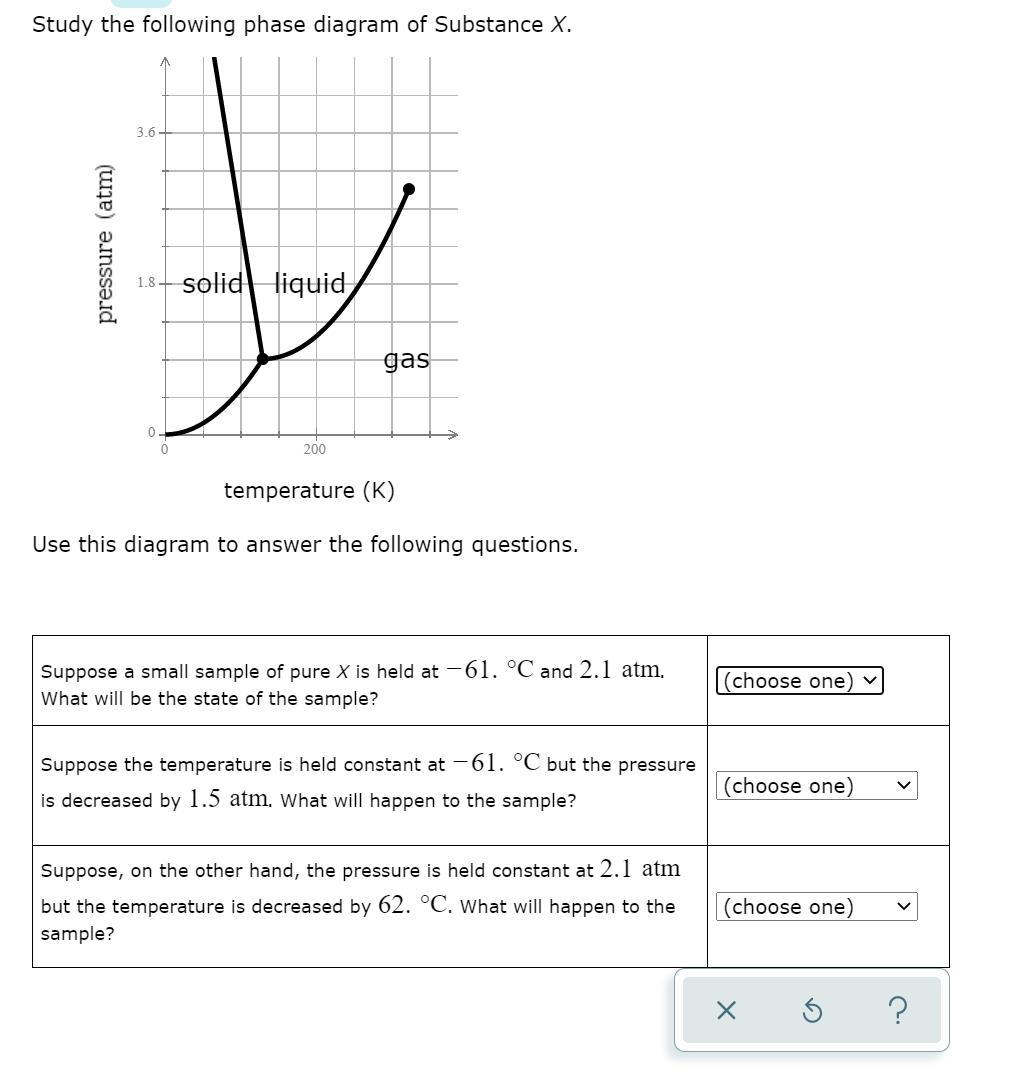
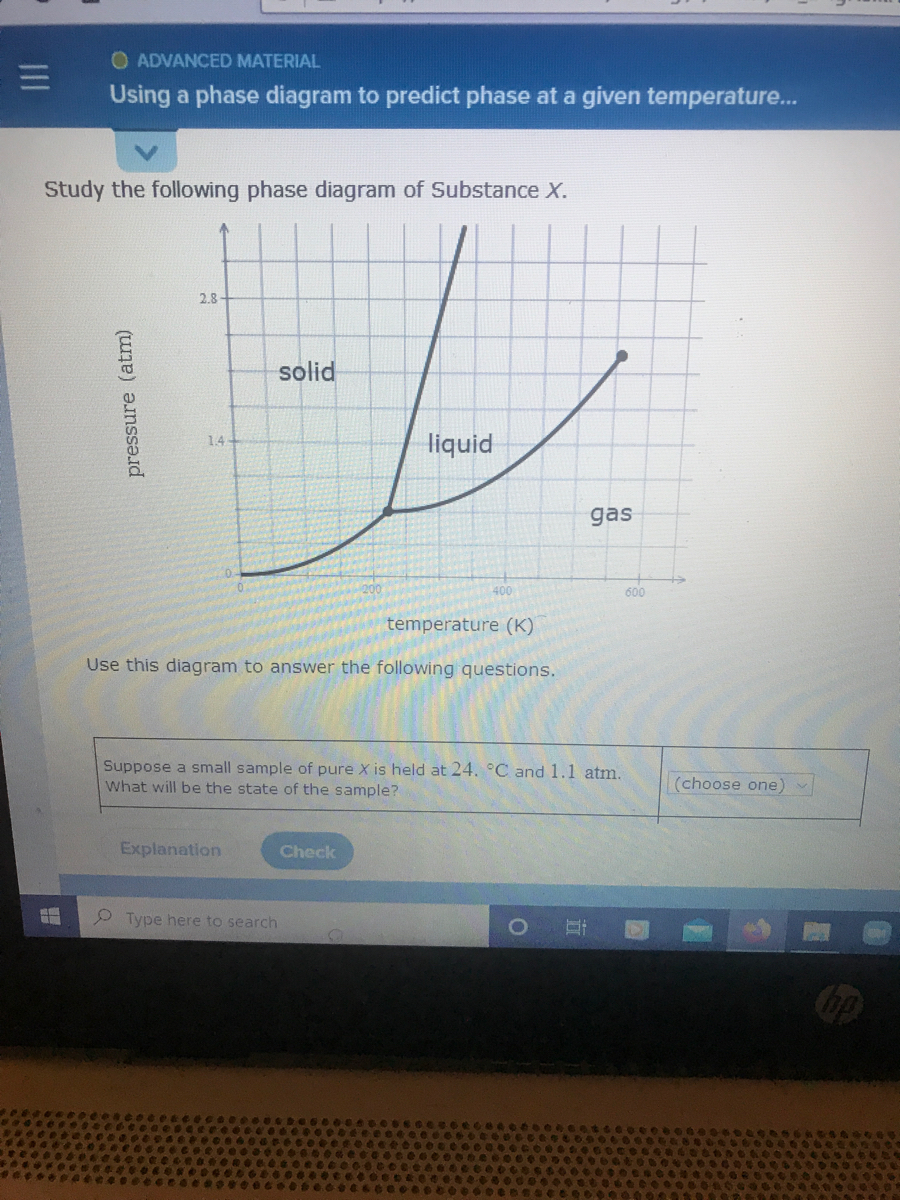
Study the following phase diagram of Subs... - Physical ... Study the following phase diagram of Substance X. solid pressure (atm) liquid gas temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -202. °C and 1.2 atm. What will be the state of the sample? (choose one) Suppose the temperature is held constant at - 202. °C but the pressure is ... Answered: Study the following phase diagram of… | bartleby Transcribed Image Text: Study the following phase diagram of Substance X. 28- solid liquid 14. gas 0. 200 400 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at 159. °C and 11.6 atm. What will be the state of the sample? (choose one) O Suppose the temperature is held constant at 159. °C but the pressure (choose one) is decreased by ... Water Phase Diagram - study.com Water Phase Diagram. A phase diagram is a graphic illustration that relates the pressure and temperature combinations responsible for the solid, liquid and gas phases of a substance. Sometimes ... Phase Diagrams: Critical Point, Triple Point ... - Study.com Phase diagrams are unique to every different substance. Let's start by looking at a phase diagram and exploring everything that's on it. The x -axis of the graph shows temperature.
Phase Diagram of Water vs Other Substances ... - Study.com Study the phase diagram of water as an example, and compare it to other substances to understand the implications of pressure and temperature changes on substance phases. Updated: 11/05/2021 ...
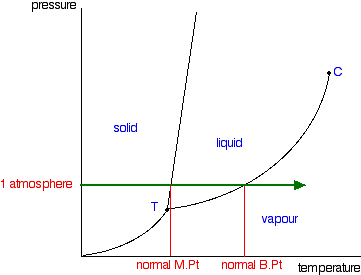
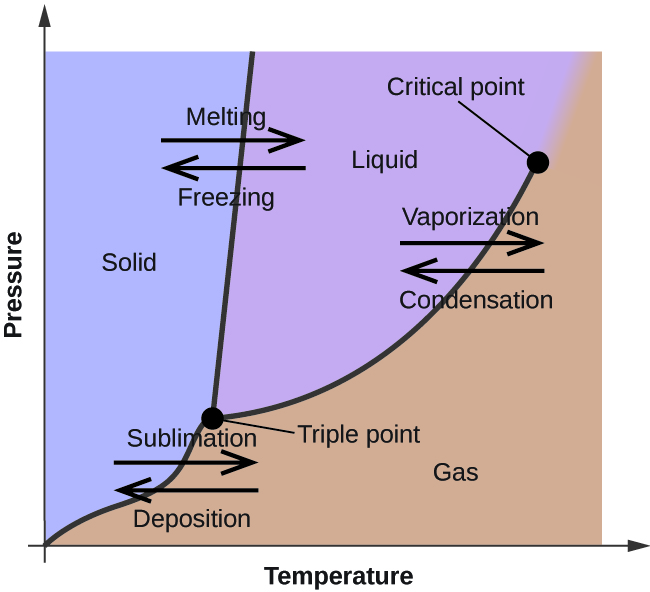
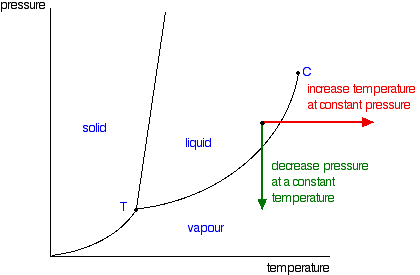
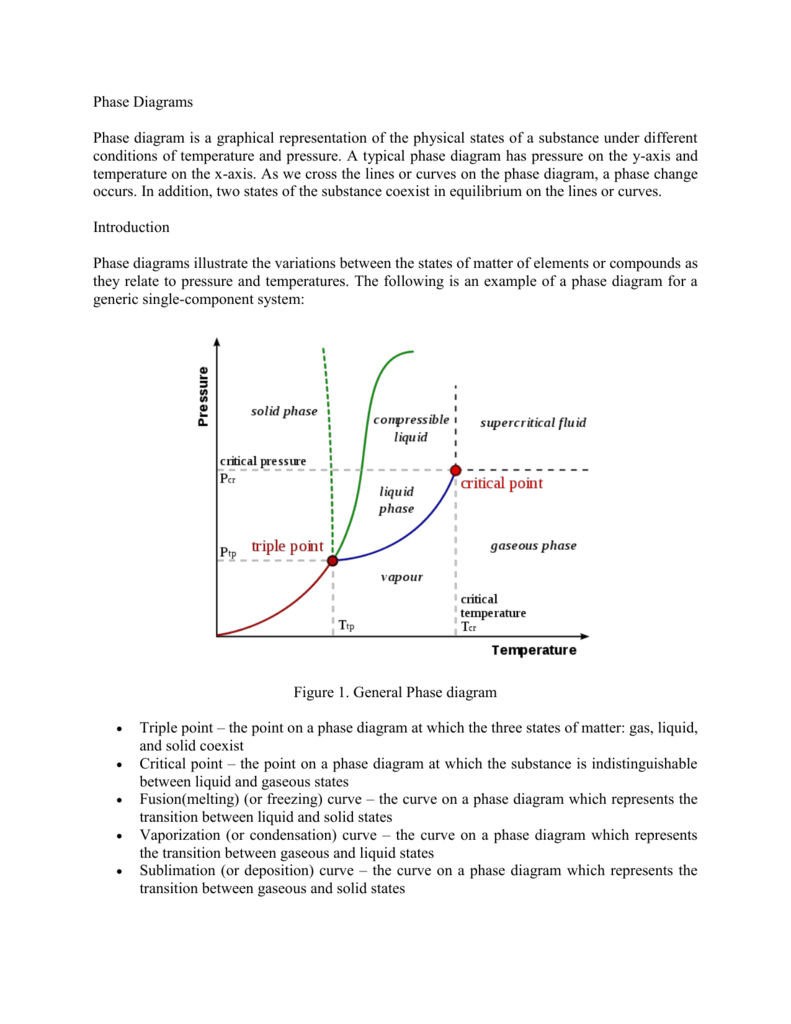
Phase Diagrams - Purdue University Phase Diagrams. The figure below shows an example of a phase diagram, which summarizes the effect of temperature and pressure on a substance in a closed container. Every point in this diagram represents a possible combination of temperature and pressure for the system. The diagram is divided into three areas, which represent the solid, liquid ...
Study the following phase diagram of Substance X. solid ... please help Study the following phase diagram of Substance X. 2.4 pressure (atm) solid liquid gas 400 600 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -9. °C and 1.1 atm.
[Solved] Study the following phase diagram of Substance X ... Study the following phase diagram of Substance X. pressure (atm) 20- solid liquid gas 200 400 600 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at - 196. .C and 19.4 atm. (choose one) + What will be the state of the sample? Suppose the temperature is held constant at - 196.
1.14 Flashcards - Quizlet The substance cannot exist in the liquid form. Which of the following occurs when the temperature and pressure are below the triple point of a substance? The solid melts to liquid and then vaporizes to gas. For a given sample of carbon dioxide (CO2) you increase the temperature from -80°C to 0°C and decrease the pressure from 30 atm to 5 atm.
Study the following phase diagram of Subs... - Physical ... Study the following phase diagram of Substance X. 1.6- pressure (atm) 0.8 solid liquid gas 200 400 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at -48. °C and 0.9 atm. What will be the state of the sample? (choose one Suppose the temperature is held constant at - 48. °C but the ...
Solved Study the following phase diagram of Substance X ... Transcribed image text: Study the following phase diagram of Substance X pressure (atm) solid liquid gas temperature (K) Use this diagram to answer the following questions, Suppose a small sample of pure X is held at 71. C and 19.2 atm. what will be the state of the sample? (choose one Suppose the temperature is held constant at 71. "C but the pressure is decreased by 15 2 atm.
1.12 Phase Diagrams Flashcards - Quizlet In the phase diagram for substance X, what is the triple point of substance X? 29 degrees C, 2.2 atm. According to this phase diagram of water, what happens when you increase the pressure from 50 kPa to 150 kPa on an ice cube at 0 degrees C? the ice cube melts. Which of the following occurs when the temperature and pressure are below the triple ...
Chemistry Unit 1 Lesson 14 Flashcards - Quizlet Chemistry Unit 1 Lesson 14. 1. Which curve or point of a phase diagram would indicate the melting point at various temperatures and pressures? 2. In the phase diagram for substance X, what is the triple point of substance X? Refer to the phase diagram for substance X in Problem Set 60: Phase Diagrams, in the Chemistry: Problems and Solutions book.
Phase Diagrams | General Chemistry - Lumen Learning Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
[Solved] Study the following phase diagram of Substance X ... Study the following phase diagram of Substance X. solid pressure (atm) 14 liquid gas 200 400 600 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at 149. "C and 20.3 atm. (choose one) : What will be the state of the sample? Suppose the temperature Is held constant at 149.
Solved Study the following phase diagram of Substance X ... 100% (14 ratings) Transcribed image text: Study the following phase diagram of Substance X. pressure (atm) ED temperature (K) Ο Which is the triple point of X? η Ι Φ Ο Which line must the temperature and pressure have crossed if a solid sample of X is observed to melt? OOOOOOOOOOOO η OoooOOOOOO Ι Φ Ο If a sample of pure X is a gas, in ...
Answered: Study the following phase diagram of… | bartleby Science Chemistry Q&A Library Study the following phase diagram of Substance X. 16- solid liquid gas 100 200 temperature (K) Use this diagram to answer the following questions. Suppose a small sample of pure X is held at What will be the state of the sample? 104. °C and 6.1 atm. (choose one) v (choose one) Suppose the temperature is held constant at - 104. °C but the pressure is increased by ...
Study the following phase diagram of Subs... - Physical ... Study the following phase diagram of Substance X. 3.2- solid pressure (atm) 16 liquid gas 100 temperature (K) Use this diagram to answer the following questions Suppose a small sample of pure X is held What will be the state of the sample? 43. °C and 2.4 atm. choose one) e Suppose the temperature is held constant at 43. °C but the pressure is ...














0 Response to "39 study the following phase diagram of substance x."
Post a Comment