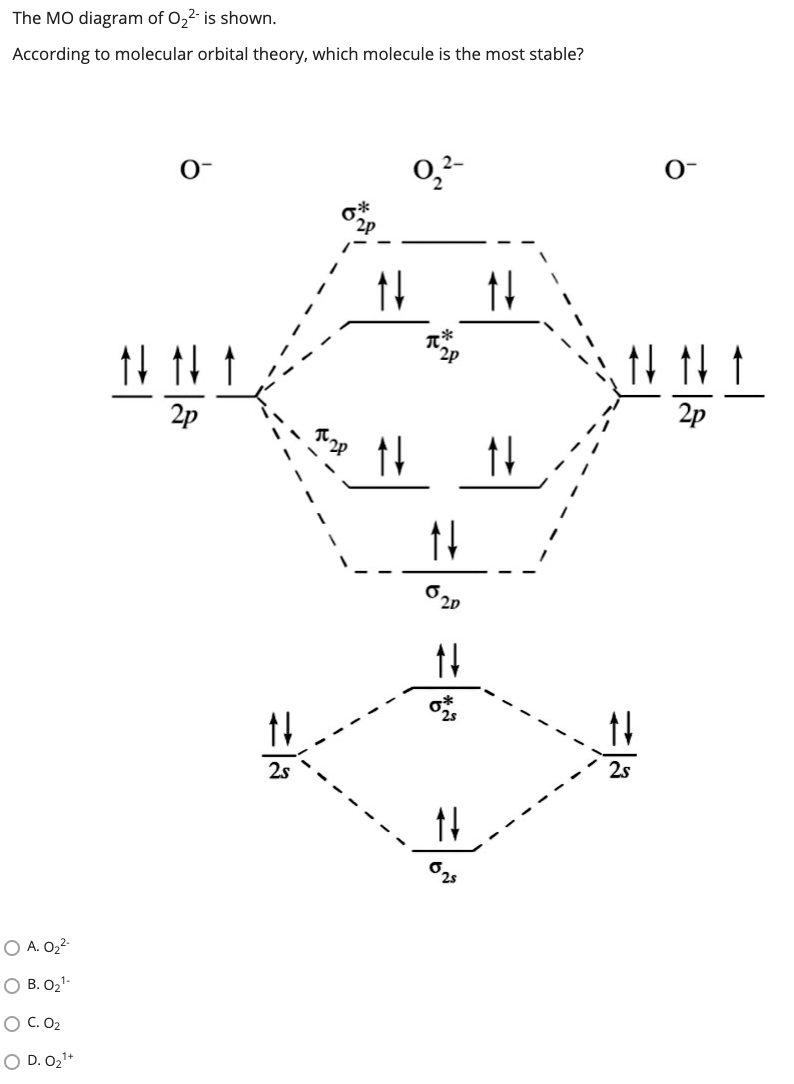
39 molecular orbital diagram for o2 2-
Chemistry Review: Exam 2 Flashcards - Quizlet a. There are more nodes found in the 2s orbital. b. Electrons in the 2s orbital are shielded by electrons in the 2p. c. The larger number of electrons found in the 2p orbital leads to greater repulsion. d. The shape of the orbital ultimately determines the energy of the electrons. e. Electrons in the 2s orbital can penetrate the 1s orbital and ... What's the MOT diagram of O2 +2 ion? - Quora Aug 25, 2017 — The Molecular orbital diagram for O2 is like this: As you can see the oxygen molecule has two unpaired electrons in the lower π* ant-bonding states.
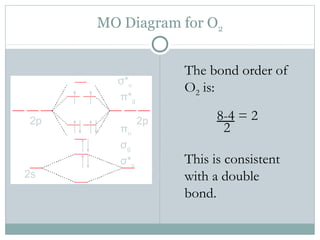
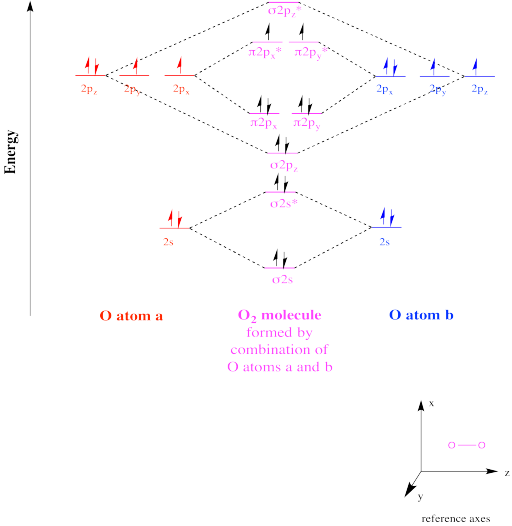
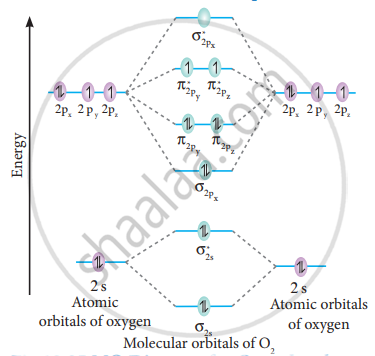
CHAPTER 5: MOLECULAR ORBITALS The Lewis structures have an unpaired electron and an average bond order of 1.5. O2 has two unpaired electrons in its π* orbitals, and a bond order of 2.29 pages
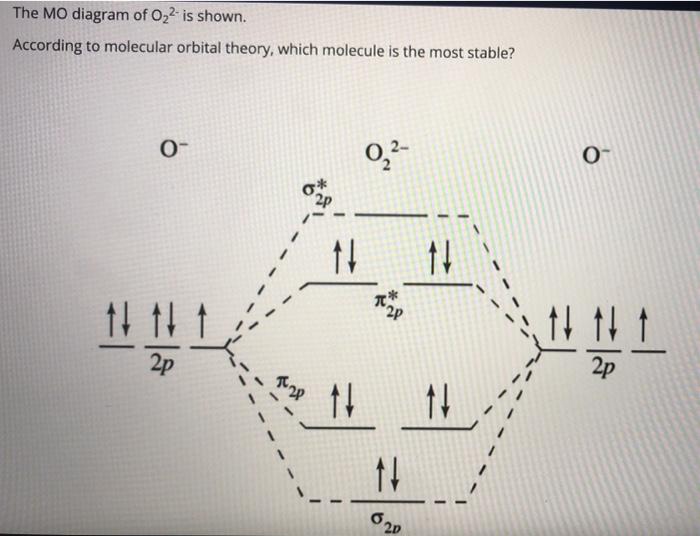
Molecular orbital diagram for o2 2-
› bond-order-for-o2Bond order for o2 - Crack Chemistry The bond order can be calculated by writing the molecular orbital diagram of the o2 molecule. Bond order for o2 can easily be solved by using the bond order trick also. We will discuss that also below. › faculty › kboudreaQuantum Numbers, Atomic Orbitals, and Electron Configurations Another way to indicate the placement of electrons is an orbital diagram ... 1s 2 2s 2 2p 4: O2-1s 2 2s 2 2p 6: ... The Molecular Nature of Matter and Change, 2nd ed ... Draw molecular orbital diagram of O2 - - or N2 - Toppr As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b − N a ] / 2 = [1 0 − 6] / 2 = 2. Therefore there is a double bond present as O = O.
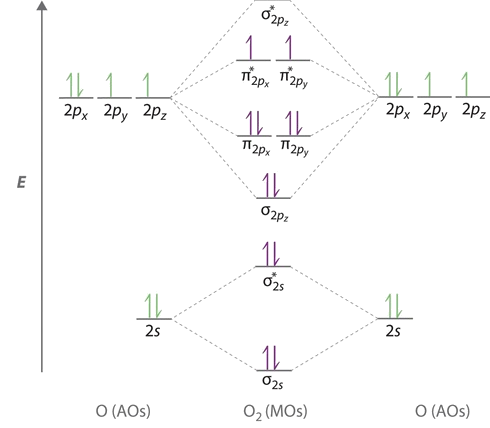
Molecular orbital diagram for o2 2-. Keith JOHNSON | Massachusetts Institute of Technology, MA ... We have calculated the crystal field parameter, 10 Dq and Racah's parameter B and C for several transition metal ions namely, V^2+, 3+, Cr^2+, 3+, 4+ and Mn^2+, 3+, 4+ with oxygen ligands in a ... SO2 Molecular Geometry, Hybridization, Lewis Structure ... SO2 Molecular Orbital Diagram. The molecular orbital diagram of SO2 is attached below: SO2 Molecular Orbital Diagram. A molecular orbital diagram gives us an idea about how the atomic orbitals of two different atoms can fuse and give rise to a new orbital. This further helps us to find out the bond order, bond length, and bond strength of any compound. In this MO we can … Oxygen - Wikipedia Orbital diagram, after Barrett (2002), ... At standard temperature and pressure, oxygen is a colorless, odorless, and tasteless gas with the molecular formula O 2, referred to as dioxygen. As dioxygen, two oxygen atoms are chemically bound to each other. The bond can be variously described based on level of theory, but is reasonably and simply described as a covalent … Molecular Orbital (MO) Diagram for O2(2+) - YouTube Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.
Molecular Orbital (MO) Diagram for O2(2-) - YouTube This is the peroxide ion, O2(2-), so you KNOW it's going to be stable.It has a bond order of 1, which also makes sense. Molecular Orbital Theory - Purdue University The molecular orbital diagram for an O 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the … quiz 4 Flashcards | Quizlet molecular supercritical metallic covalent-network . Molecular _____ solids consist of atoms or molecules held together by dipole-dipole forces, London disperson forces, and/or hydrogen bonds. a. Ionic b. Molecular c. Metallic d. Covalent-network e. Metallic and covalent-network. b. Crystalline solids _____. a. have their particles arranged randomly b. have highly ordered … Energy level diagram for Molecular orbitals - Chemical ... 20/03/2019 · N b = 2 , Na =0. Bond order = 1. Positive value of bond order indicates that H 2 molecule is stable.. Bond order value of 1 means that two hydrogen atoms are connected by a single bond.. Greater value of bond order for H 2 molecule than H 2 + ion shows that two H 2 molecule is more stable than H 2 +.. Bond length of H 2 is smaller than that of H 2 + ion.. As no …
By writing molecular orbital configuration for NO,CO,O2 ... 18/03/2018 · Also see here... Bond order for "NO"^+ Order by bond length: "NO", "NO"^(+), "NO"^(-) Is "CO" a Lewis acid? "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The … Draw the molecular orbital energy diagram for oxygen molecule (O2 ... Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), ... SO2 Lewis Structure, Hybridization, Molecular Geometry ... 01/03/2022 · Now let’s learn the last topic of this article, the molecular orbital diagram of SO2. SO2 Molecular Orbital Diagram . The molecular orbital diagram of SO2 is attached below: A molecular orbital diagram gives us an idea about how the atomic orbitals of two different atoms can fuse and give rise to a new orbital. This further helps us to find out the bond order, bond … Bond order ofO2 O2+ O2 and O22 is in order A O2 langle ... - Vedantu $\text{Bond order =}\dfrac{\text{1}}{\text{2}}\l... Read More. Related Questions. According to Molecular orbital theory which of the following is correct ...
In the molecular orbital diagram for O2^ + ion, the highest occupied ... σ MO orbital · π MO orbital · π∗ MO orbital · σ∗ MO orbital · As it can be seen from the given structures that in the molecular orbital diagram for O2+ ion, the ...
› 40078040 › James_E_Brady_The(PDF) James E. Brady The Molecular Nature of Matter (6th ... James E. Brady The Molecular Nature of Matter (6th Edition) Copia. Download. James E. Brady The Molecular Nature of Matter (6th Edition) Copia
MO Diagram for O2(2-) - YouTube It is sigma2s(2)sigma2s*(2)sigma2p(2)pi2p(4)pi2p*(4)Bond order 1. It is stable. In fact, it's the perioxide ion.
Molecular docking, validation, dynamics simulations, and ... 08/09/2020 · 2.9. Molecular dynamics simulations. Molecular dynamics simulations were performed using the Desmond package for the following complexes i) Free-protein, ii) Protein co-crystallized with N3 peptide inhibitor, and iii) Top three best low binding energy ligand-protein complexes after docking. All the complexes were solvated individually by placing them in an …
Compare the stabilities of O2 , O2-,O22 - NextGurukul Oct 28, 2014 ... Ans: The stabilities of these can be best explained using Molecular orbital theory. ... Atomic orbitals of oxygen combine to form molecular ...
Draw molecular orbital diagram of O2 - - or N2 - Toppr As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b − N a ] / 2 = [1 0 − 6] / 2 = 2. Therefore there is a double bond present as O = O.
› faculty › kboudreaQuantum Numbers, Atomic Orbitals, and Electron Configurations Another way to indicate the placement of electrons is an orbital diagram ... 1s 2 2s 2 2p 4: O2-1s 2 2s 2 2p 6: ... The Molecular Nature of Matter and Change, 2nd ed ...
› bond-order-for-o2Bond order for o2 - Crack Chemistry The bond order can be calculated by writing the molecular orbital diagram of the o2 molecule. Bond order for o2 can easily be solved by using the bond order trick also. We will discuss that also below.

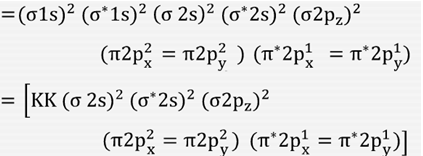




















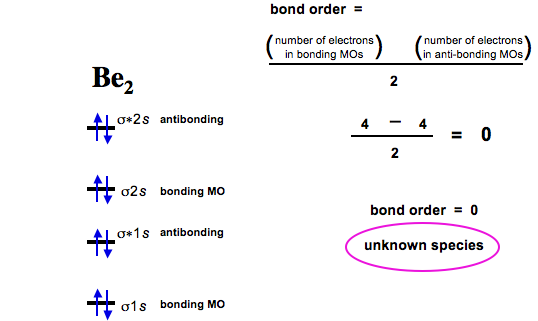


0 Response to "39 molecular orbital diagram for o2 2-"
Post a Comment