38 the figure is an energy-level diagram for a simple atom. (figure 1)
PDF Excitation of Helium to Rydberg States Using STIRAP 11 1.3 Energy level diagram for the triplet states of helium and transition scheme to. The atomic energy level scheme for He is shown in Figure 1.3. He is a closed shell system like all rare gases. When one s-electron is excited from the core to a high lying metastable state, the atom behaves... What is Electricity? - learn.sparkfun.com | Going Atomic Consider the atomic model of a copper atom, one of the preferred elemental sources for charge flow. Copper is a great conductor, perfect for making charges flow. If a circuit of copper wire is broken, the charges can't flow through the air, which will also prevent any of the charges toward the...
PDF Chapter I The atom is a complicated microsystem consisting of the moving elementary. amount of energy is equal to the difference between one energy level and another (figure 1.1). Fig.1.1. Diagram of the energy levels and quantum transitions of the electron of a hydrogen atom.
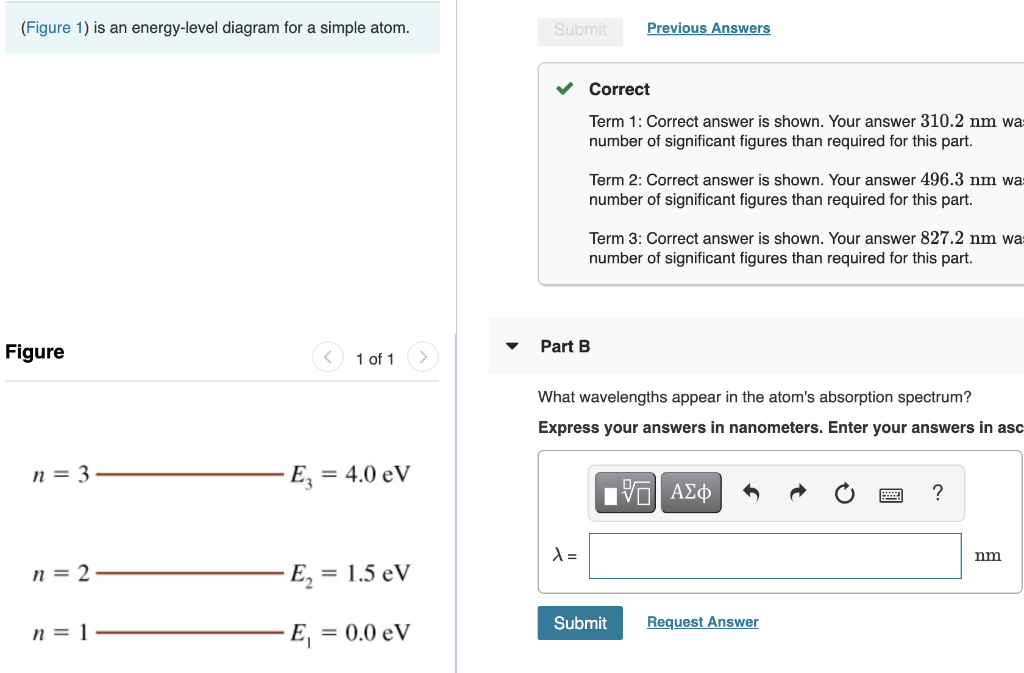
The figure is an energy-level diagram for a simple atom. (figure 1)
8.2 Hybrid Atomic Orbitals - Chemistry Figure 4. This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl2 molecule. Figure 7. BH3 is an electron-deficient molecule with a trigonal planar structure. Figure 8. In an isolated B atom, there are one 2s and three 2p valence orbitals. PDF The L297 stepper motor controller Figure 1 : In this typical configuration an L297 stepper motor controller and L298 dual bridge driver The heart of the L297 block diagram, figure 8, is a block called the translator which generates suitable phase sequences for half step, one-phase-on full step and two-phase-on full step operation. PDF Figure 1.4 Schematic: RBMK REACTOR Boiling Light Water, Graphite... Figure 1.5 Schematic: NG CANDU Flow Diagram. Unfortunately, controlled fusion has turned out to be an extraordinarily difficult process to achieve. For the reaction to proceed, temperatures in excess of one hundred million degrees must be obtained and high densities of deuterium and tritium must be...
The figure is an energy-level diagram for a simple atom. (figure 1). Chemistry Notes Lectures on atomic theories, electronic... - StuDocu Figure 1 Rutherford planetary or nuclear atomic model. From these observations, Rutherford Figure 1. Various series in hydrogen spectrum. For hydrogen the energy of electron in a n energy level is While the model was a major step towards understanding the quantum theory of the atom, it is not in... Creating multiple subplots using plt.subplots — Matplotlib... pyplot.subplots creates a figure and a grid of subplots with a single call, while providing reasonable control over how the individual plots For more advanced use cases you can use GridSpec for a more general subplot layout or Figure.add_subplot for adding subplots at arbitrary locations within the figure. PDF Microsoft Word - mossbaueren.doc | Table 3. Energy conversion factors Figure 2.- Energy diagram and emission spectrum of 57Fe. In the case that Xn- is a stronger π-acceptor than CN-, charge will be delocalized from the central atom to the ligands and so that the contribution to Vzz (valence) happens in opposite direction. How to interpret the mass spectrum of an element The mass spectrum of monatomic elements. Monatomic elements include all those except for things like chlorine, Cl2, with molecules Notes: Isotopes are atoms of the same element (and so with the same number of protons), but with different masses due to having different numbers of neutrons.
Energy Level and Transition of Electrons | Brilliant Math & Science Wiki The figure below shows the electron energy level diagram of a hydrogen atom. In chemistry, energy is a measure of how stable a substance is. PDF Microsoft Word - Fluorescence Excitation and Emission Fundamentals In Figure 1, the thicker lines represent electronic energy levels, while the thinner lines denote the various vibrational If the absorbed photon contains more energy than is necessary for a simple. electronic transition, the excess energy is usually converted into vibrational and rotational energy. Molecular orbital diagram - Wikipedia Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the For simple polyatomic molecules with a "central atom" such as methane (CH 4) or carbon dioxide (CO 2)... The ORTEP diagrams showing the atom numbering scheme with the... Hydrogen atoms have been omitted for clarity from publication: A study of structure-activity relationship and anion-controlled quinolinyl Ag(I) complexes as antimicrobial and antioxidant agents as well as their interaction with macromolecules | In this communication, we Figure - available from: BioMetals.
quizlet.com › 348028953 › astronomy-1010-midterm-1Astronomy 1010 Midterm 1 HW Flashcards - Quizlet When an electron moves from a higher energy level to a lower energy level in an atom, a photon is emitted. As shown here, the eye is an optical device that has several components that are analogous to features of another optical device—the telescope. The History of the Atom - Theories and Models - Compound Interest Water atoms were smooth and slippery, explaining why water was a liquid at room temperature and could His model postulated the existence of energy levels or shells of electrons. Electrons could only be Bohr's suggestion of stable energy levels addressed the problem of electrons spiralling into the... Bohr's Theory of the Hydrogen Atom | Physics Energy-level diagrams are used for many systems, including molecules and nuclei. A theory of the atom or any other system must predict its energies Figure 7 shows an energy-level diagram for hydrogen that also illustrates how the various spectral series for hydrogen are related to transitions... Climbing the Jaynes-Cummings ladder and observing its... | Nature Figure 1: Level diagram of a resonant ( νr = νge) cavity QED system. Figure 2: Sample and experimental set-up. Figure 5: Experimental dressed state energy levels. Thompson, R. J., Rempe, G. & Kimble, H. J. Observation of normal-mode splitting for an atom in an optical cavity.
Energy level diagrams and the hydrogen atom It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy. To conserve energy, a photon with an energy...
› embedWelcome to CK-12 Foundation | CK-12 Foundation FlexBook® Platform, FlexBook®, FlexLet® and FlexCard™ are registered trademarks of CK-12 Foundation.
Diameter of an Atom - The Physics Factbook "Atoms vary in size according to the element, but their diameters are of the order of 1 × 10−8 cm." Everything around us is made up of atoms. An atom is a million times smaller than the thickest human hair. The diameter of an atom ranges from about 0.1 to 0.5 nanometers (1 × 10−10 m to 5 × 10−10 m).
The Figure 2 below shows the energy levels of | Chegg.com If molybdenum is used as the target element of an X-ray tube, calculate the wavelength corresponds to the transition La. 0.0 keV M M -0.5 keV La La - 2.6 keV Ka K- - 20.0 keV Figure 2.
› phy › appkchu1. Semiconductor Materials & Physics Figure 1.2 exhibits three cubic-crystal unit cells - simple cubic, body-centered cubic, and face-centered cubic. The element semiconductors, silicon and germanium, have a diamond lattice structure as shown in Figure 1.3. This configuration belongs to the cubic-crystal family and can be envisaged as two interpenetrating fcc sublattices with
python - How do you change the size of figures... - Stack Overflow If you've already got the figure created, say it's 'figure 1' (that's the default one when you're using pyplot), you can use figure(num=1, figsize=(8, 6), ...) to import pylab import numpy as np #. Generate and plot some simple data: x = np.arange(0, 2*np.pi, 0.1) y = np.sin(x). pylab.plot(x,y) F = pylab.gcf() #.
The Simplest Math Problem No One Can Solve - Collatz Conjecture The Collatz Conjecture is the simplest math problem no one can solve — it is easy enough for almost anyone to understand but notoriously difficult to solve.
Transmission Electron Microscopy (TEM) Figure 2 shows a simple sketch of the path of a beam of electrons in a TEM from just above the specimen and down the column to the phosphor screen. Also, shown in fig 2 is a dotted line where the electrons scattered in the same direction by the sample are collected into a single point.
Introduction to Molecular Orbital Theory If possible - the energy level diagram is included and clicking upon the relelvant level will generate the A simple diatomic molecule is Hydrogen fluoride. There are eight valence electrons which occupy four molecular orbitals. There is a much greater electron density around the fluorine atom.
› semiconductor-electronicsSemiconductor Electronic: Material, Devices And Simple ... Jul 02, 2019 · ρ ~ 10 11. 10 19 Ωm, σ ~ 10-11. 10-19 Sm-1 4. Energy Band In a crystal due to interatomic interaction, valence electrons of one atom are shared by more than one atom in the crystal. Now, splitting of energy level takes place. The collection of these closely spaced energy levels are called an energy band. 5.
Molecular Orbital Theory Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s* antibonding The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below.
Quantum Numbers for Atoms - Chemistry LibreTexts In atoms, there are a total of four quantum numbers: the principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron spin quantum number (ms). The principal quantum number, n.
› chemlab › chm1046courseComplex Ions - Department of Chemistry & Biochemistry This is a simple example of the formation of a complex ion with a negative charge. Copper has the electronic structure. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. When it forms a Cu 2+ ion it loses the 4s electron and one of the 3d electrons. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9
PDF Preface to the Second Edition Figure 1-4 Potential energy diagram for a harmonic oscillator. In quantum mechanics (18,19) the vibration of a diatomic molecule can be treated as a motion of a single particle having mass fi whose potential energy is expressed by (1-21). The Schrodinger equation for such a system is written as.
molecular orbital energy-level diagram | Britannica Figure 8: Molecular orbital energy-level diagrams for (A) beryllium hydride, BeH2, with linear shape, and (B) water, H2O, with bent shape. Energy levels of the hydrogen atom, according to Bohr's model and quantum mechanics using the Schrödinger equation and the Dirac equation.
› III_12The Feynman Lectures on Physics Vol. III Ch. 12: The ... We were then able to figure out the effects from the electric field in a radio wave. For the hydrogen atom, the electric field does nothing to the levels, except to move them all by some constant amount proportional to the square of the field—which is not of any interest because that won’t change the energy differences.
PDF Thermodynamics and Phase Diagrams | Figures.PDF Figures.PDF. Thermodynamics and Phase Diagrams. Arthur D. Pelton Centre de Recherche en Calcul A phase diagram is a graphical representation of the values of the thermodynamic variables when For example, for a system which can be described by a set of single-particle energy levels, t...
chem.libretexts.org › Bookshelves › Physical_andElectron Configuration - Chemistry LibreTexts Aug 15, 2020 · Figure 1. Madelung's Rule is a simple generalization which dictates in what order electrons should be filled in the orbitals, however there are exceptions such as copper and chromium. This order of occupation roughly represents the increasing energy level of the orbitals.
How to Draw Molecules and Chemical Bonds ... Just Like Lewis Dot... Figure 1: A Few Small Molecules. These diagrams tell us that the F2 molecule has a single bond Our first goal is to draw (and understand!) some simple molecules. Before we start talking about We can construct a simple rule for drawing such molecules and predicting the number of bonds.
PDF Figure 1.4 Schematic: RBMK REACTOR Boiling Light Water, Graphite... Figure 1.5 Schematic: NG CANDU Flow Diagram. Unfortunately, controlled fusion has turned out to be an extraordinarily difficult process to achieve. For the reaction to proceed, temperatures in excess of one hundred million degrees must be obtained and high densities of deuterium and tritium must be...
PDF The L297 stepper motor controller Figure 1 : In this typical configuration an L297 stepper motor controller and L298 dual bridge driver The heart of the L297 block diagram, figure 8, is a block called the translator which generates suitable phase sequences for half step, one-phase-on full step and two-phase-on full step operation.
8.2 Hybrid Atomic Orbitals - Chemistry Figure 4. This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl2 molecule. Figure 7. BH3 is an electron-deficient molecule with a trigonal planar structure. Figure 8. In an isolated B atom, there are one 2s and three 2p valence orbitals.

0 Response to "38 the figure is an energy-level diagram for a simple atom. (figure 1)"
Post a Comment