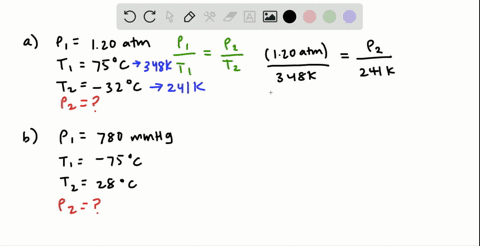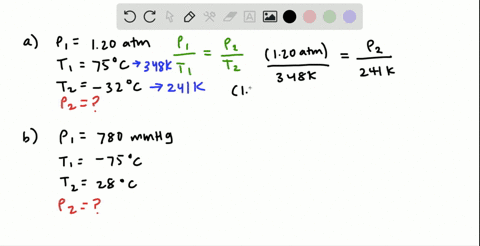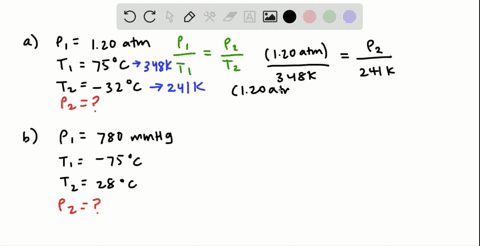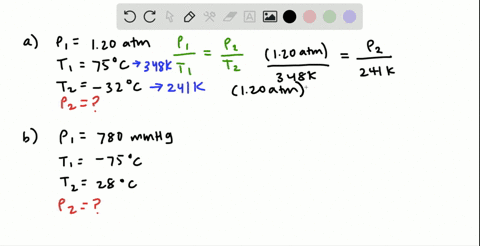
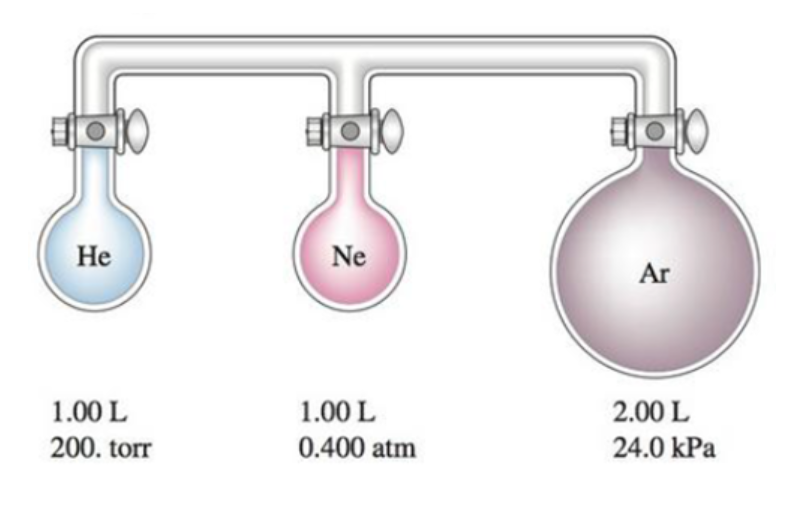
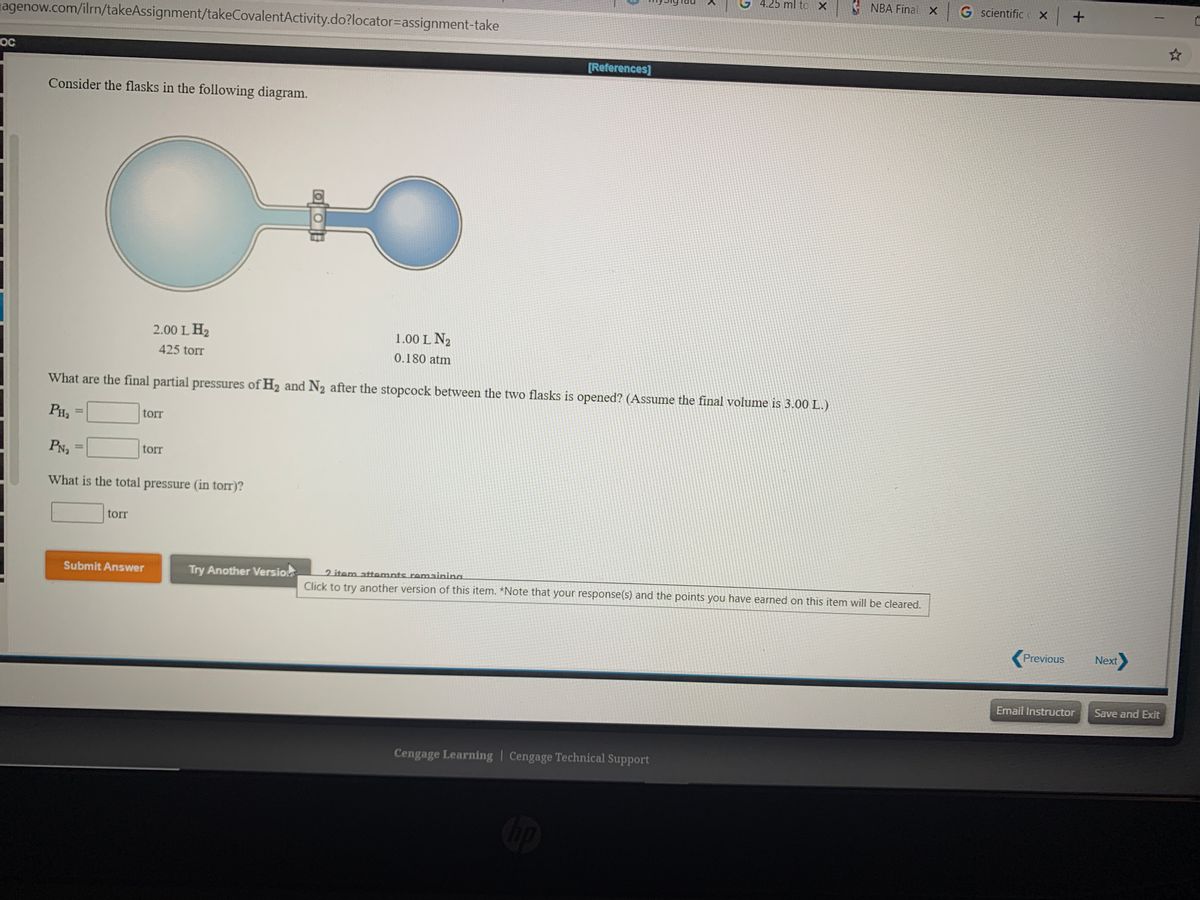
38 consider the flasks in the following diagram. what are the final partial pressures of h2 and n2
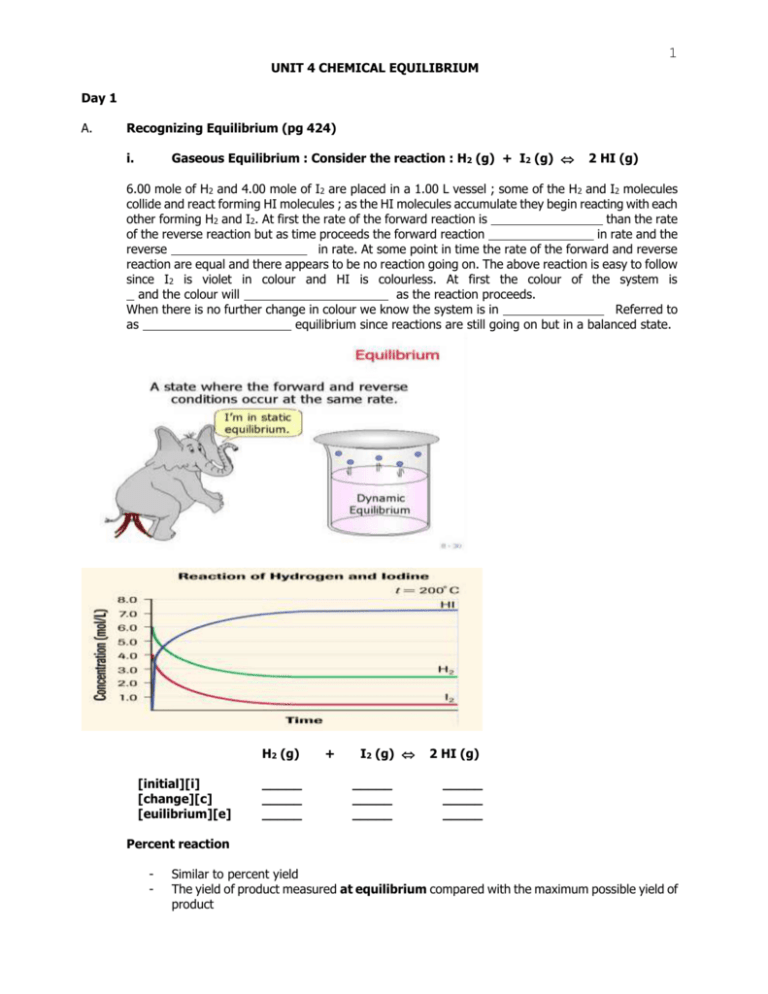
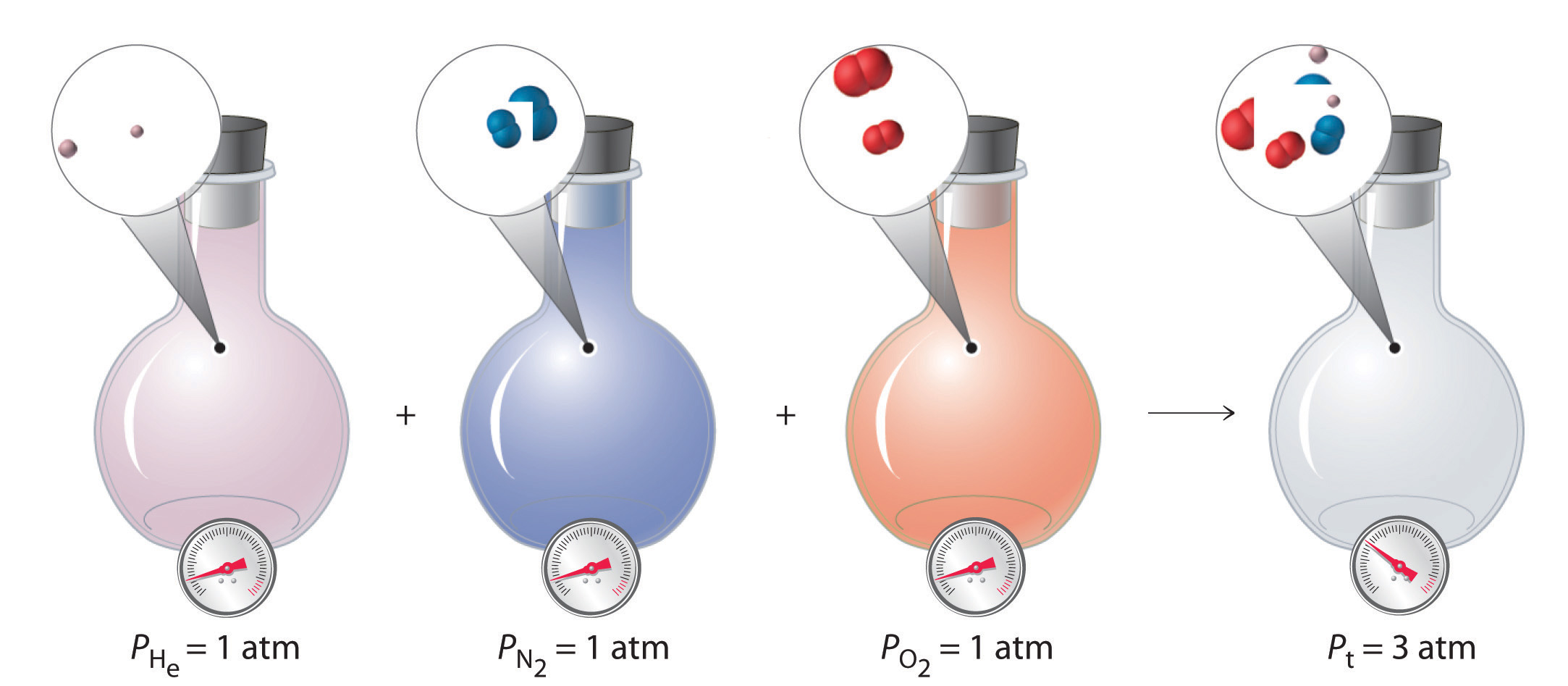
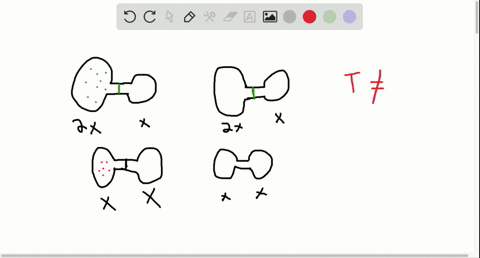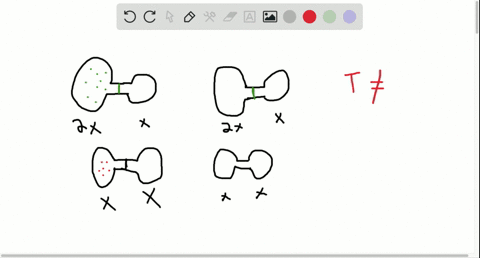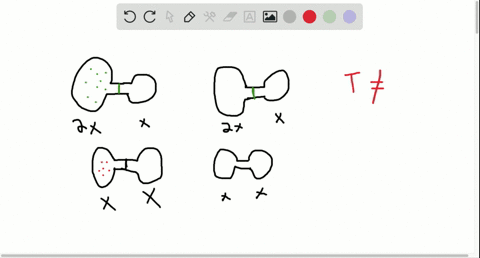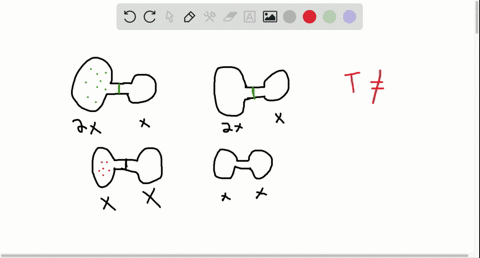
Consider the flasks in the following diagram. 2.50 L 1.00 L 405 ... Tour were asked to calculate the partial pressures of each gas and the total ... atm What are the final partial pressures of H2 and N2 after the stopcock ...4 answers · Top answer: so that we have to flash a flask of pure helium that is to 75 mL and 752 tour and a flask ... Chemistry - Page 238 - Google Books Result Steven S. Zumdahl, Susan A. Zumdahl · 2013 · ScienceConsider the flasks in the following diagrams. ... What are the final partial pressures of H2 and N2 after the stopcock between the two flasks is opened?
Chemistry: An Atoms First Approach - Page 362 - Google Books Result Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste · 2020 · ScienceConsider the flasks in the following diagram. What are the final partial pressures of H2 and N2 after the stopcock between the two flasks is opened?

Consider the flasks in the following diagram. what are the final partial pressures of h2 and n2
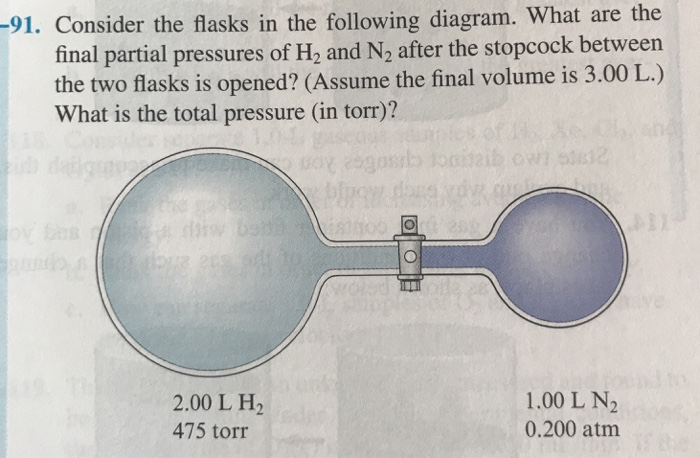
GasLaws-wkst-key.pdf - Chemistry 301 Consider the flask diagramed below with the following pressures 492 torr for H2 and 0.376 atm for N2. What are the final partial pressures of H2 and N2 ...3 pages Consider the flasks in the following diagram. What are the final ... Consider the flasks in the following diagram. What are the final partial pressures of H2 and N2 after the stopcock between the two flasks is opened?1 answer · Top answer: Gases - System: energy (Temperature); intensity (pressure); composition (mole fraction) - Mole fraction is proportional to partial pressure n - χ = component ... 91. Consider the flasks in the following diagram. | Chegg.com Consider the flasks in the following diagram. What are the final partial pressures of H2 and N2 after the stopcock between the two flasks is opened? (Assume the ...1 answer · Top answer: HOPE TH...
Consider the flasks in the following diagram. what are the final partial pressures of h2 and n2. Can you help me with this AP Chemistry gas laws problem? Oct 10, 2017 — Consider two flasks, the first flask has 2.00L of hydrogen at a pressure of 475 torr, the second has 1.00 L of nitrogen at a pressure of ...1 answer · Top answer: For such a problem, you can use Boyle's law of constant temperature. P1V1 = P2V2. Just apply this to each gas separately and add the pressures at the ... Exercise 5.3 10. Consider the flasks in the following diagram. What are the final partial pressures of H₂ and N₂ after the stopcock between the two flasks is opened.2 pages Consider two flasks. What are the final partial pressure of H2 ... Apr 6, 2017 — Assume the final volume is 3.00 L. What is the total pressure in torr? Flask 1: 2.00 L H2, 475 torr. Flask 2: 1.00 L N2, 0.200 atm3 answers · Top answer: It's 317+50.7 torr = 368 torr 91. Consider the flasks in the following diagram. | Chegg.com Consider the flasks in the following diagram. What are the final partial pressures of H2 and N2 after the stopcock between the two flasks is opened? (Assume the ...1 answer · Top answer: HOPE TH...
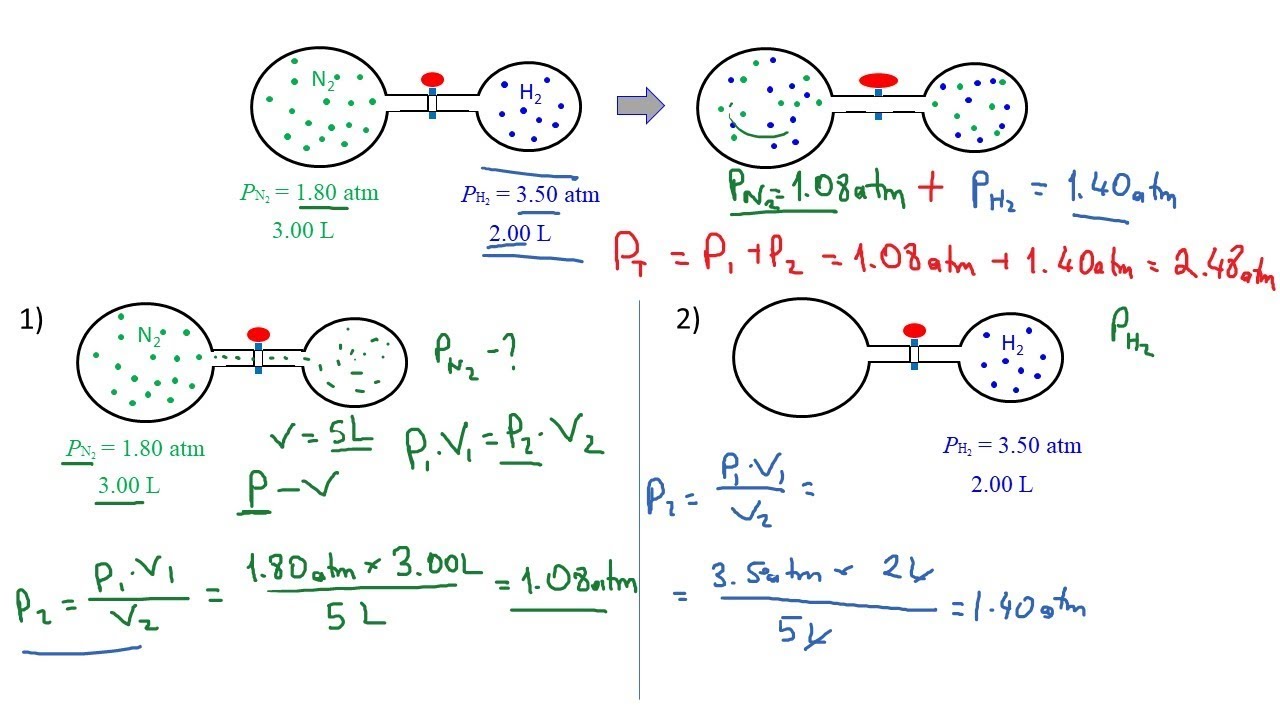
Consider the flasks in the following diagram. What are the final ... Consider the flasks in the following diagram. What are the final partial pressures of H2 and N2 after the stopcock between the two flasks is opened?1 answer · Top answer: Gases - System: energy (Temperature); intensity (pressure); composition (mole fraction) - Mole fraction is proportional to partial pressure n - χ = component ... GasLaws-wkst-key.pdf - Chemistry 301 Consider the flask diagramed below with the following pressures 492 torr for H2 and 0.376 atm for N2. What are the final partial pressures of H2 and N2 ...3 pages





![Solved] Consider the flasks in the following diagrams. a ...](https://s3.amazonaws.com/si.question.images/image/images7/442-C-P-c-G(22).png)

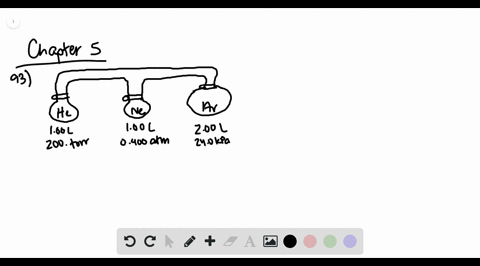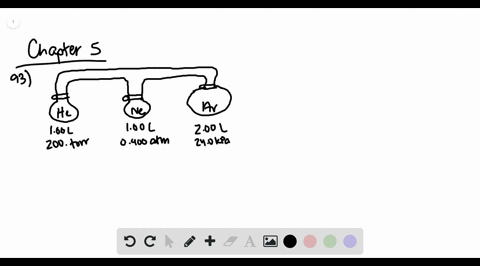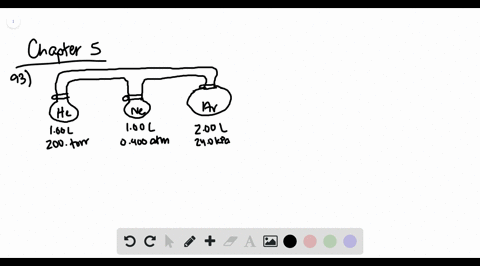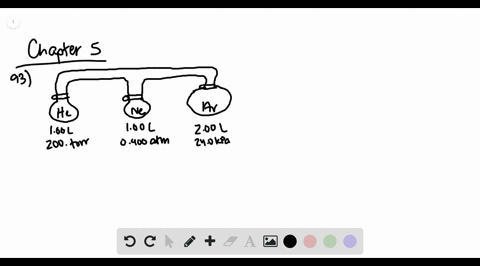
![Solved [References] Consider the flasks in the following ...](https://media.cheggcdn.com/media%2Fd2e%2Fd2e514c7-f25a-46ba-87df-42e2f08e21d2%2Fphp7oWZuL.png)
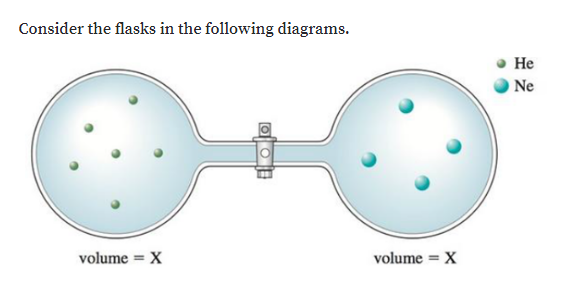
0 Response to "38 consider the flasks in the following diagram. what are the final partial pressures of h2 and n2"
Post a Comment