40 reaction coordinate diagram endothermic
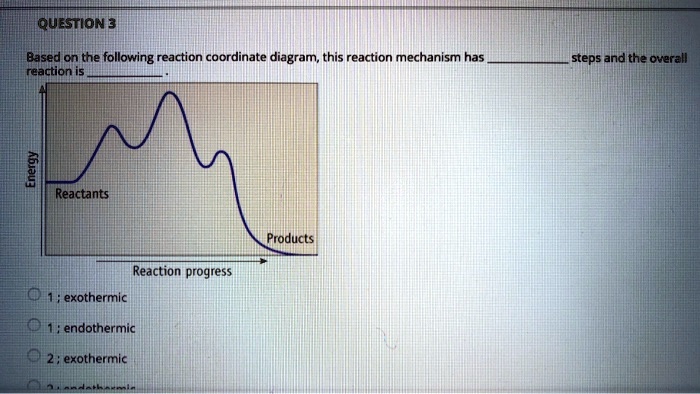
NEB New Syllabus (Curriculum) of Class 11 and 12 Chemistry ... 2020-05-12 · General characteristics of halogens; Comparative study on preparation (no diagram and description is required); Chemical properties [with water, alkali, ammonia, oxidizing character, bleaching action] and uses of halogens (Cl 2, Br 2 and I 2); Test for Cl 2, Br 2 and I2 ; Comparative study on preparation (no diagram and description is required), properties ( … 6. Reaction Coordinate Diagram - VIZISCIENCE® INTERACTIVE LABS 6. Reaction Coordinate Diagram. Given the following reaction, sketch a reaction coordinate graph. The reaction involves two steps, step 1 is the slowest step and step 2 is the fastest step. Both steps are exothermic. Indicate on the diagram the overall enthalpy change of the reaction, the reaction for the transition states and intermediate states.
Solved Potential Energy Reaction Coordinate The forward ... Chemistry. Chemistry questions and answers. Potential Energy Reaction Coordinate The forward reaction that this diagram describes is Select one: exothermic O a. O b. endothermic O c. neither exothermic nor endothermic O d. there is not enough information to tell, because the axes are not labeled with proper units Check.

Reaction coordinate diagram endothermic
An endothermic reaction with high activat... - Physical ... An endothermic reaction with high activation energy for the forward reaction is given by the diagram : 4 Potential energy Potential energy (1) (2) Reaction coordinate Reaction coordinate Potential energy Potential energy (3) (4) Reaction coordinate Reaction coordinate Answer 1 Introduction to Catalysis - Wiley-VCH uct P separates from the catalyst in an endothermic step. 2 1 Introductionto Catalysis. 3 bonding reaction separation reaction coordinate + A B catalyst catalyst A B catalyst P catalyst P potential energy A B P Figure 1.2. Potential energy diagram of a heterogeneous catalytic reaction, with gaseous reactants and products and a solid catalyst. Note that the uncatalyzed … 04.02 Reaction Coordinate Diagrams and Stability Trends ... General structure of a reaction coordinate diagram, including transition states and intermediates. Overall free energy change and activation energy. Definiti...
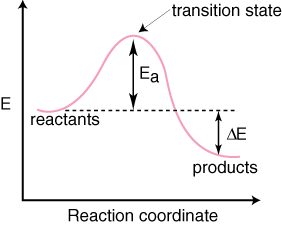
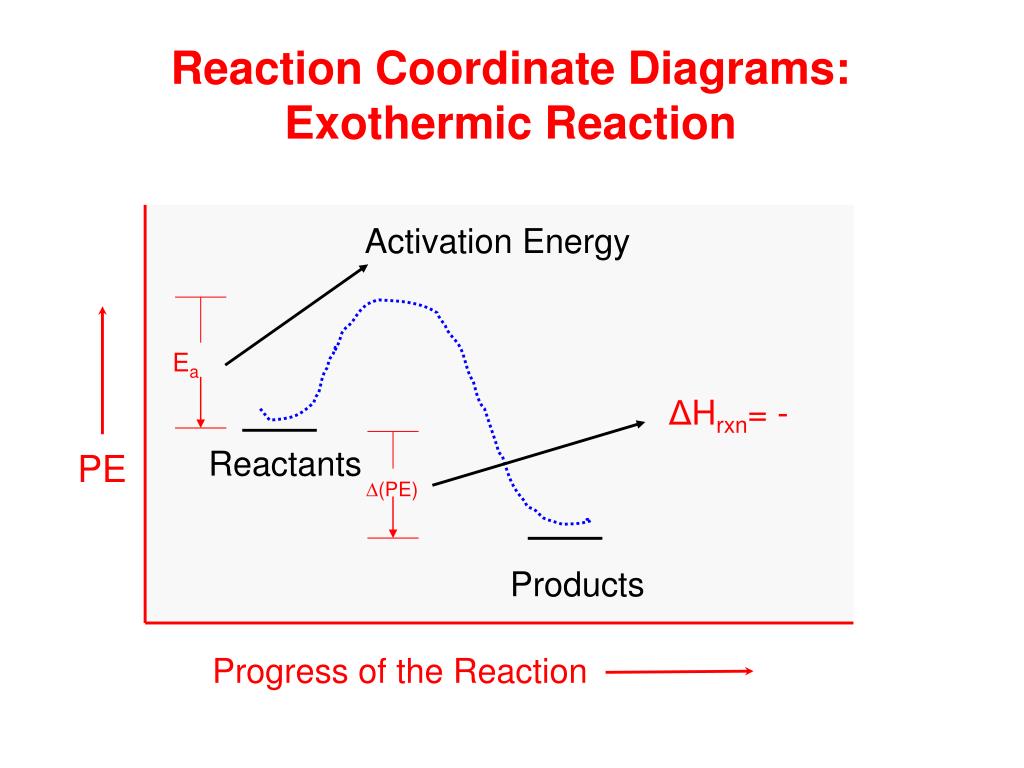
Reaction coordinate diagram endothermic. What are Endothermic Reactions? (with Examples & Video) Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. PDF Chapter 5. Reactions of Alkenes and Alkynes Learning ... Reaction coordinate diagram describes the energy changes that takes place during a reaction Important Terminologies: starting materials, products, reaction mechanism, energy diagram, reaction coordinate, heat of reaction ( H), exothermic reaction, endothermic reaction, activation energy (E a) or free energy of activation ( G‡), reaction intermediate, Essentials of Physical Chemistry by B.S ... - Academia.edu Preface The Essentials of Physical Chemistry has been written for BSc students. It has been national bestseller for more than 65 years. It has been used by more than 2 million students. It is 26 editions old. It really has been that long. A lot of PDF Kinetics Notes - Commack Schools For endothermic reactions, ones in which energy is absorbed, the potential energy of the products is higher than the ... The diagram below shows the reaction coordinate for a reversible catalyzed and uncatalyzed reaction. Referring to the diagram, answer the questions that follow. 2. 5. 6. 8. 9. The reaction shown above is (a) endothermic,
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... Answered: Q1: For the following energy diagrams,… | bartleby Solution for Q1: For the following energy diagrams, identify the reaction as endothermic or exothermic, and determine AEan and Ea. 21 E4 Reaction coordinate (PDF) Inorganic Chemistry Housecroft | Yurika Almanda ... Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link. Analyzing Energy With a Reaction Coordinate Diagram ... A reaction coordinate diagram is a graph that plots energy versus reaction progress. The amount of energy that needs to be added is called the activation energy , which is the point where the line ...
Endothermic Reaction Coordinate Diagram - Wiring Diagrams A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other. Let's consider a general reaction where a reactant or set of reactants, A, or set of products, B. The diagram below is called a reaction coordinate diagram. B is at a lower total ... PDF EnergyDiagrams - Towson University • Energy Diagrams are a plot of the reacon steps,&or"ReaconCoordinate"(Xaxis)versus theEnergy(KcalorKJ) ENERGY REACTION COORDINATE • Ina spontaneous&reacon,&theproduct(s)are more stable than the reactant(s),& thus the ... REACTION COORDINATE NON-SPONTANEOUS OR ENDOTHERMIC The following reaction coordinate diagram ... The following reaction coordinate diagram represents Energy an endothermic reaction an exothermic reaction a reaction in which catalyst is used a reaction that is neither endothermic or exothermic 1 See answer Advertisement Advertisement saraswatigiri44 is waiting for your help. Add your answer and earn points. PDF Thermodynamics vs Kinetics - Columbia University A general Reaction Coordinate Diagram relating the energy of a system to its geometry along one possible reaction pathway is given in the figure below. In the figure below, the Activation Energy, Ea is that critical minimum energy in a chemical reaction required by reactants to be converted into products. the quantities, Ea;
Which answer defines exothermic reaction?, exothermic ... Endothermic reaction : A chemical reaction which is accompanied by the absorption of energy is called an endothermic reaction. C (s) + 2S (s) + Heat → CS 2 (l); Exothermic reaction : A chemical reaction which is accompanied by the release of energy is called exothermic reaction.
Reaction Coordinate Diagram Endothermic - Wiring Diagrams A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. The " reaction coordinate " plotted along the abscissa represents the diagrams can describe both exothermic and endothermic reactions.A reaction will be exothermic if the energy of the products is less than the energy of the reactants.
Arrhenius Theory and Reaction Coordinates The key is that there are many many potential paths between reactants and products. The reaction coordinate represents the lowest energy path. For example, in the reaction of CH 3 Cl + OH- to form CH 3 OH and Cl-, the mechanism of this reaction is a single step in which the CH 3 Cl collides with the OH- and forms the products. We can envision a reaction coordinate for this reaction which is the lengthening of the C-Cl bond (the one that is breaking) and the shortening of the C-O bond (the ...
physical chemistry: bonding Flashcards - Quizlet Coordinate/dative (covalent) If wrong CE = 0/3 but if 'covalent' or left top line blank, mark on. (Lone) pair of electrons/both electrons (on F-) CE if lone pair is from B Donated from F-/fluoride or donated to the BF3 Must have the - sign on the F ie F-Ignore Fl-M3 dependent on M2. State the bond angle in the BF4- ion. 109° to 109.5° An ultrasound imaging agent has the formula …
Reaction Coordinate Diagram Endothermic Vs Exothermic 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O! A reaction with ∆H°0 is endothermic. Figure 8: Reaction Coordinate Diagrams showing favorable or unfavorable and slow or fast reactions [7] The relative stability of reactant and product does not define the feasibility of any reaction all by itself.
PDF Exothermic Endothermic "downhill" "uphill" 7. Be able to recognize and read potential energy diagrams. Reaction Coordinate Reaction Coordinate Exothermic Endothermic "downhill" "uphill" 8. ∆H is (+) for endothermic reactions and is (-) for exothermic reactions. 9. The rates of the forward and reverse reactions are equal at equilibrium. 10.
Is Melting Exothermic or Endothermic? - Techiescientist The given diagram is called a reaction coordinate diagram. In this, the energy of species is plotted as a function of reaction progress. It represents an exothermic reaction. For example, respiration is an exothermic process. Respiration is defined as the oxidation of food to release energy. Since energy is released, it is an exothermic process.
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In endothermic reactions, there is less energy in the reactants than ...
Solution Manual Brady Chemistry 6TH Edition PDF | PDF ... Reactants: 6 N, 42 H, 2 P, 20 O, 3 Ba, 12 C; Products: 3 Ba, 2 P, 20 O, 6 N, 42 H, 12 C; The reaction is balanced . 1.6. Review Questions 1.1. This answer will be student dependent. 1.2. Observation, testing and explanation. 1.3 (a) (b) (c) A law is a description of behavior based on the results of many experiments which are true while a theory is a tested explanation of the …
PDF imarkic.weebly.com tables to determine if this reaction is endothermic or exothermic. Construct a reaction coordinate diagram that shows the endothermic or exothermic nature of the reaction and illustrates why this reaction is under kinetic control. Each diagram below (I, Il, Ill, IV) describes a possible reaction: 2 AB(g) A2(g) + B2(g)
Reaction coordinate - Wikipedia Diagram of a catalytic reaction, showing the energy niveau as a function of the reaction coordinate. For a catalyzed reaction, the activation energy is lower. In chemistry , a reaction coordinate [1] is an abstract one-dimensional coordinate which represents progress along a reaction pathway.
PDF Reactions of Alkenes - University of Texas at Austin • Energy diagram: A graph showing the changes in energy that occur during a chemical reaction. • Reaction coordinate: A measure in the change in positions of atoms during a reaction. Reaction coordinate Energy Energy Diagrams 6 • Transition state ‡: - An unstable species of maximum energy formed during the course of a reaction.
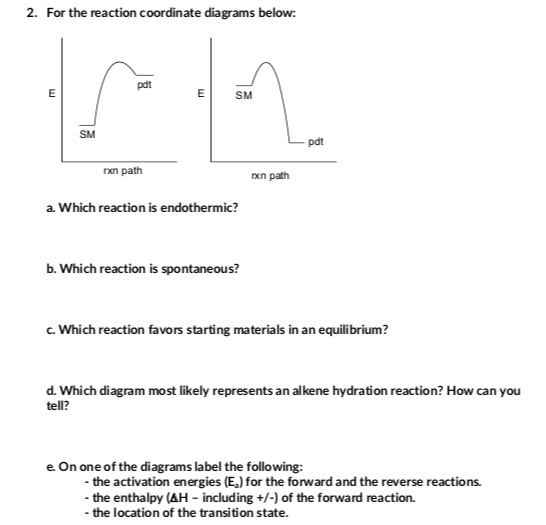
Answered: 1. a) Write the reaction profile… | bartleby Solution for 1. a) Write the reaction profile (reaction coordinate diagram) for an endothermic reaction that occurs via a 3 step mechanism with the second step…
Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction
Reaction Coordinate Diagrams Reaction Coordinate Diagram of Ozone Photolysis The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction . Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
Reaction Coordinate Diagrams - College Chemistry The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants.
Chemical equilibrium - Wikipedia In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction.The reaction rates of the …
Acid dissociation constant - Wikipedia In the case of VO 2 + (aq.), the vanadium is octahedral, 6-coordinate, whereas vanadic acid is tetrahedral, 4-coordinate. This means that four "particles" are released with the first dissociation, but only two "particles" are released with the other dissociations, resulting in a much greater entropy contribution to the standard Gibbs free energy change for the first reaction than for the …
Endothermic Reaction Coordinate Diagram - schematron.org Sep 16, 2018 · The " reaction coordinate " plotted along the abscissa represents the diagrams can describe both exothermic and endothermic reactions. A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. The fully filled in reaction coordinate diagram is ...
Match RIT to Concepts chemical change / chemical reaction Conservation of Mass and Matter: ... / heating substances / mass (amount of matter) / mixing substances Effects of Force on Motion: air resistance / force diagram / friction / macroscopic object / minimize / stability / sum of forces Energy Forms: kinetic energy / stored (potential) energy / temperature as average kinetic energy of particles of matter ...
04.02 Reaction Coordinate Diagrams and Stability Trends ... General structure of a reaction coordinate diagram, including transition states and intermediates. Overall free energy change and activation energy. Definiti...
1 Introduction to Catalysis - Wiley-VCH uct P separates from the catalyst in an endothermic step. 2 1 Introductionto Catalysis. 3 bonding reaction separation reaction coordinate + A B catalyst catalyst A B catalyst P catalyst P potential energy A B P Figure 1.2. Potential energy diagram of a heterogeneous catalytic reaction, with gaseous reactants and products and a solid catalyst. Note that the uncatalyzed …
An endothermic reaction with high activat... - Physical ... An endothermic reaction with high activation energy for the forward reaction is given by the diagram : 4 Potential energy Potential energy (1) (2) Reaction coordinate Reaction coordinate Potential energy Potential energy (3) (4) Reaction coordinate Reaction coordinate Answer




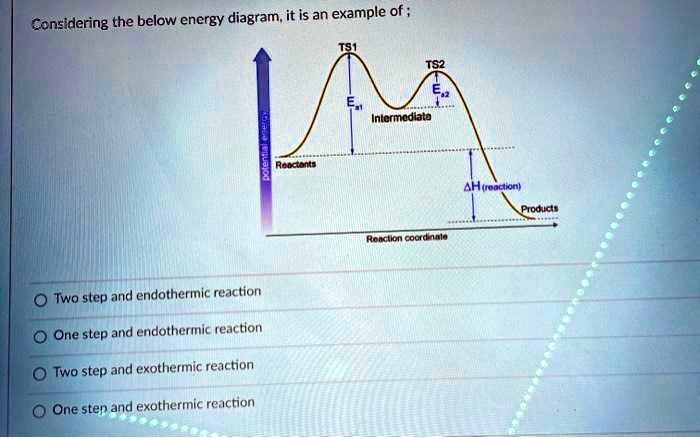





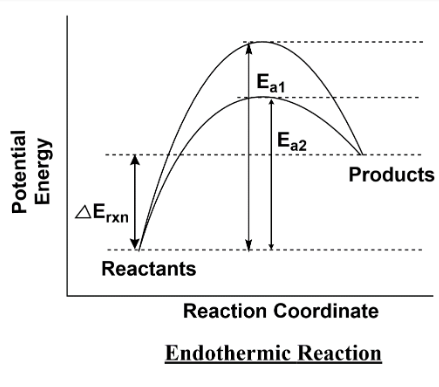







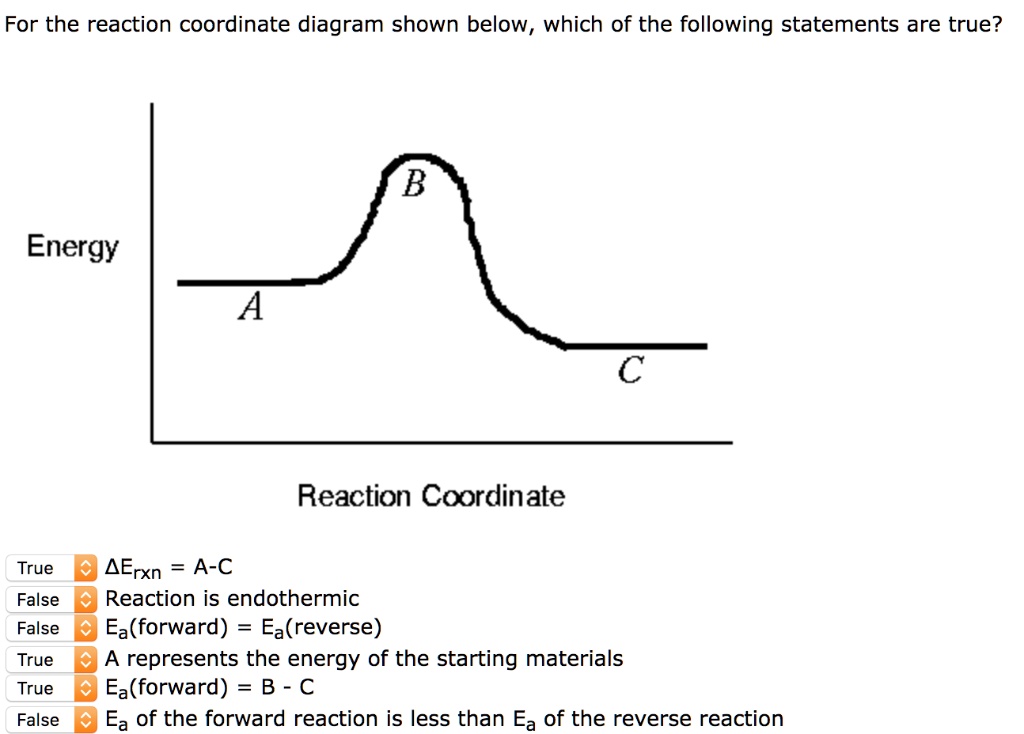



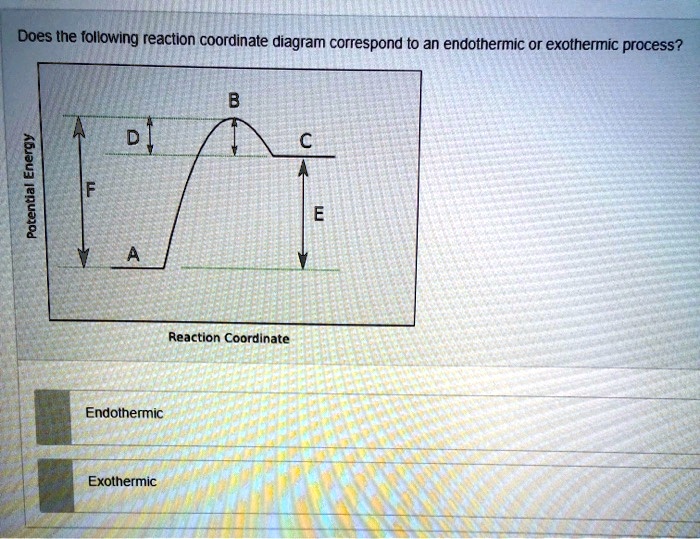







0 Response to "40 reaction coordinate diagram endothermic"
Post a Comment