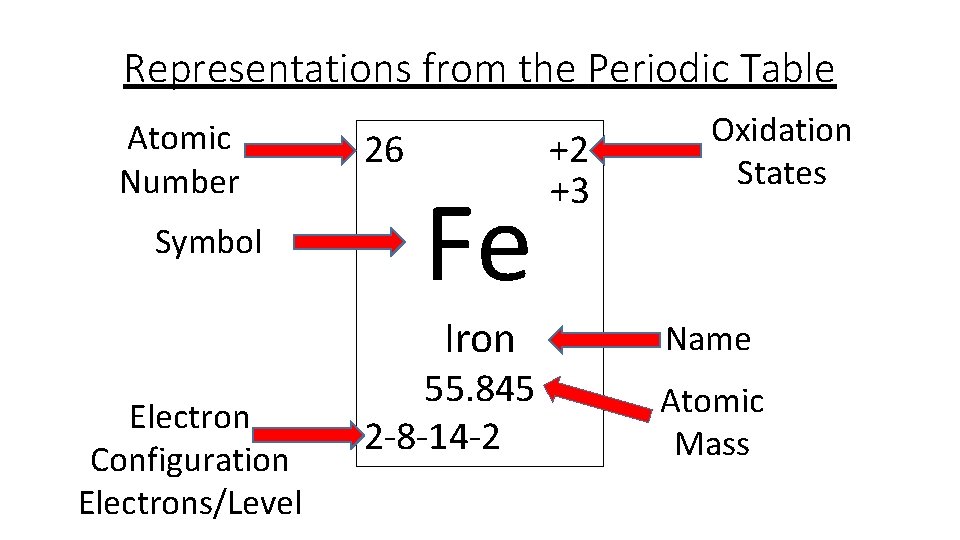
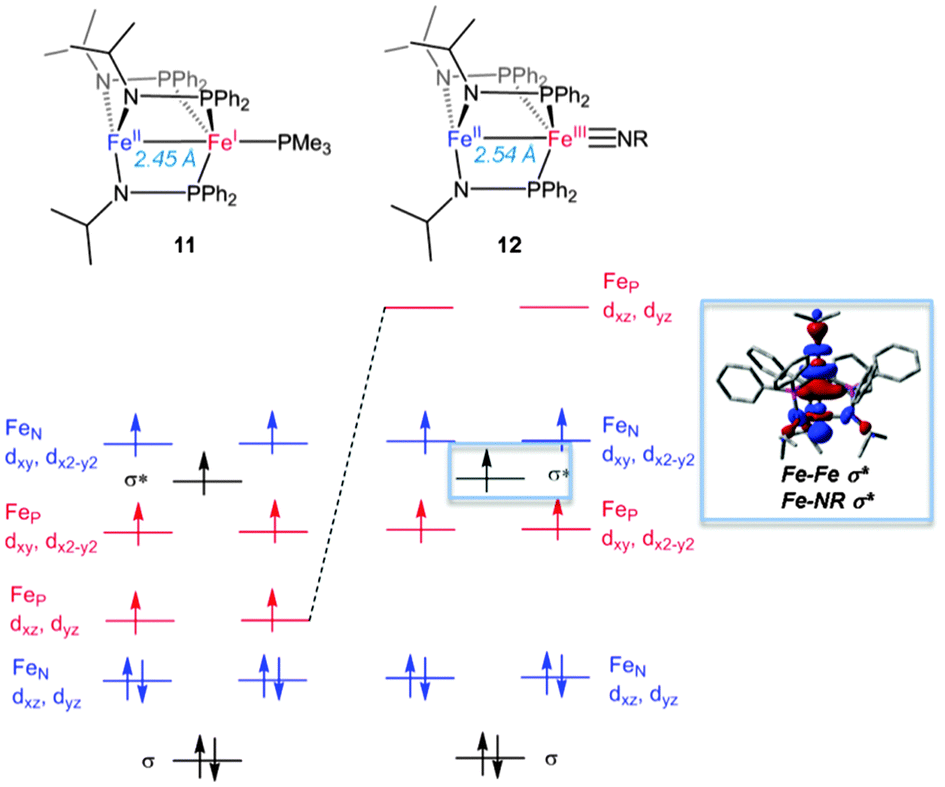
40 orbital diagram for fe
Orbital Notation for Iron (Fe) - YouTube Orbital Notation for Iron (Fe). Mr. Causey shows you step by step how to write the orbital notation for iron (Fe). for more ... PDF Molecular orbital (SCF-Xc-SW) theory of Fe2+-Mn3+, Fe3 ... Fe(t2s ) Fig.2. Molecular orbital diagram for the Fe3*Mn2*O,o clus-ter in the (a) ferromagnetic and (b) antiferromagnetic configu-rations. Orbitals indicated with a dashed line are unoccupied. Note that the orbital energies correspond to "orbital electronega-
Fe2+ Orbital Diagram - Wiring Diagrams A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.electron configuration for Fe2+ - CHEMISTRY COMMUNITYMolecular orbital diagram - Wikipedia
Orbital diagram for fe
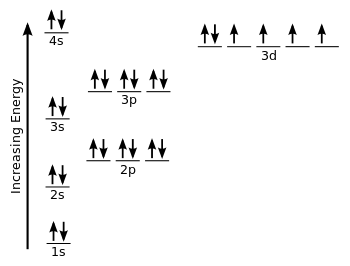
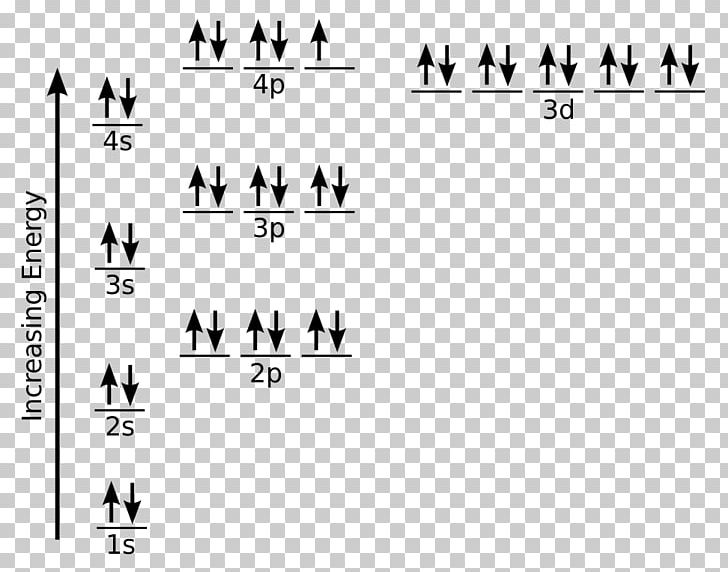
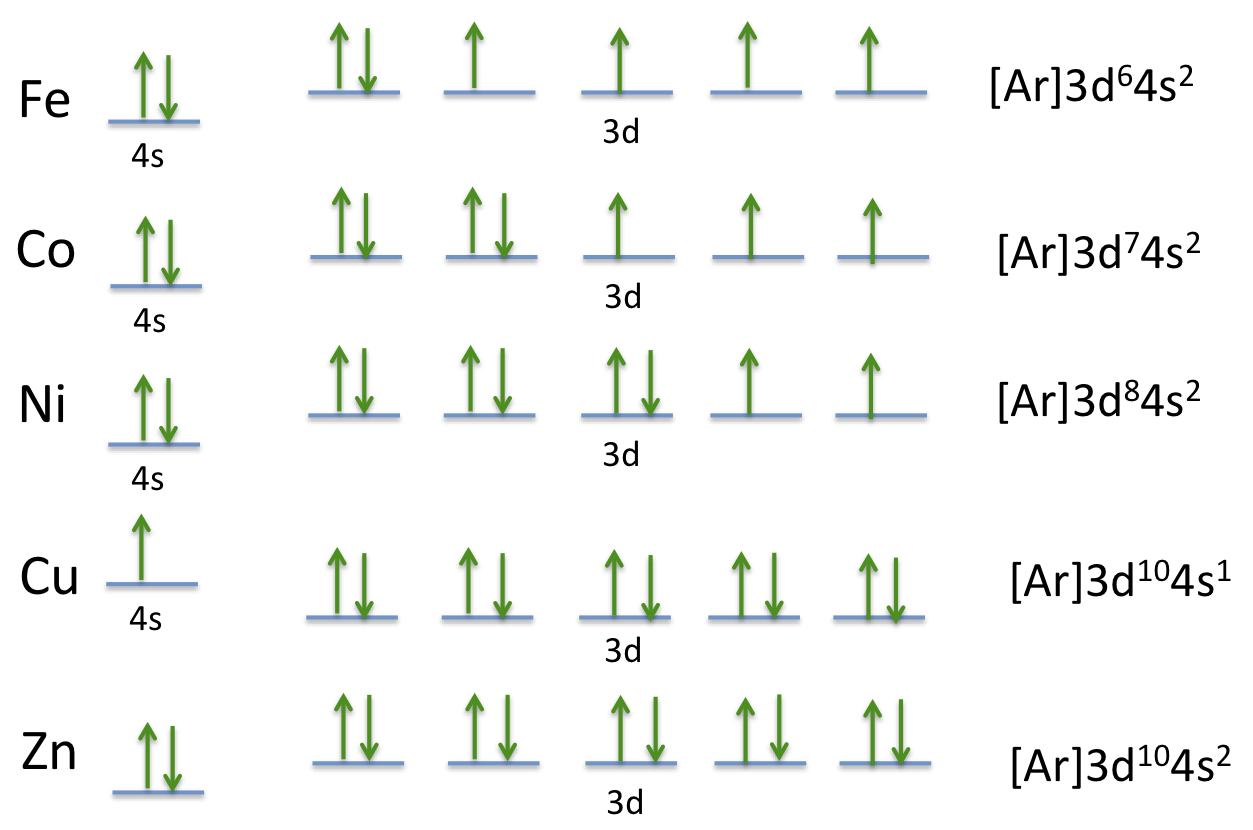
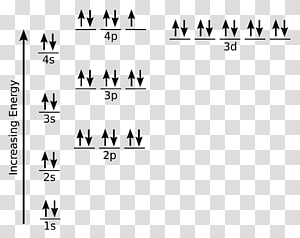
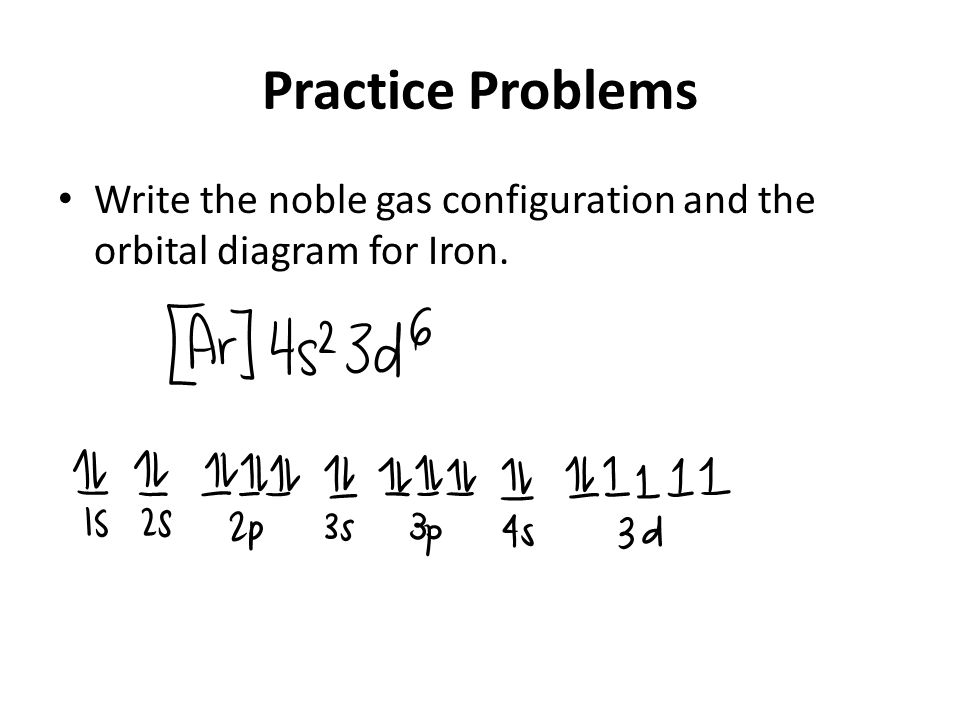
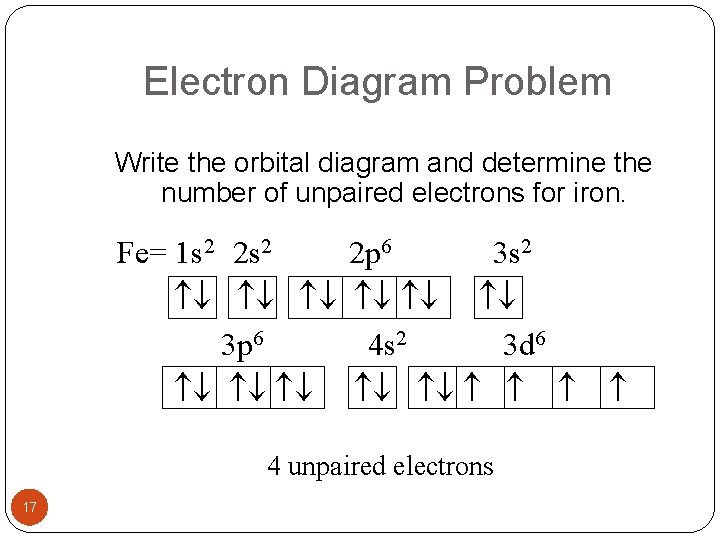
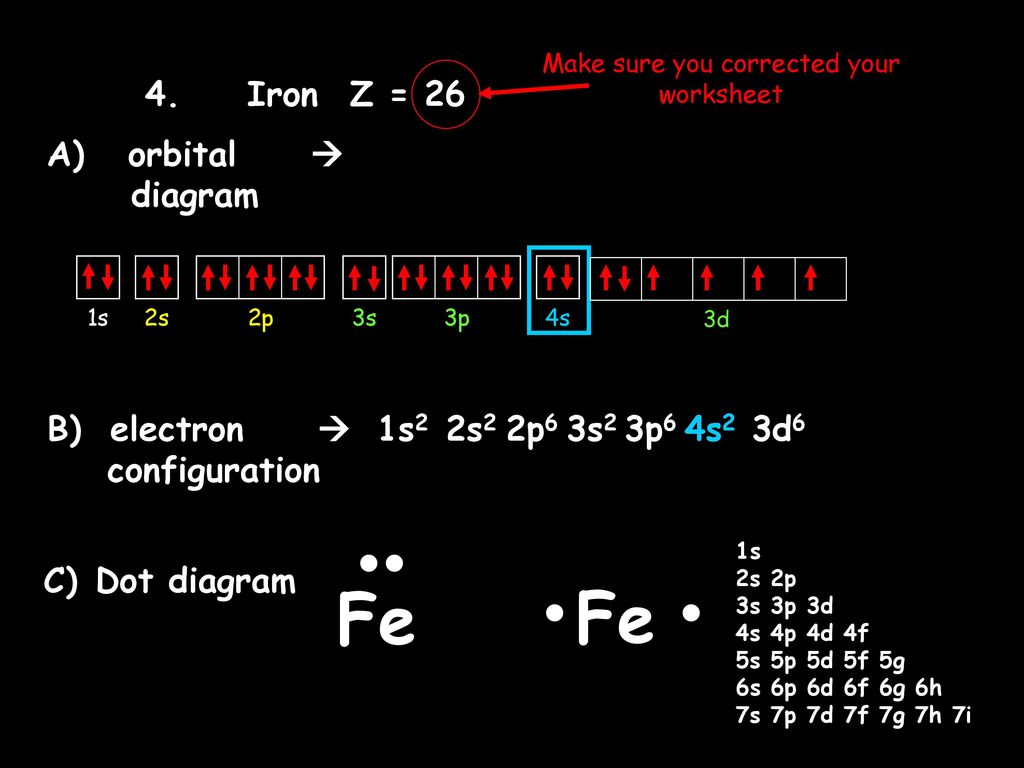
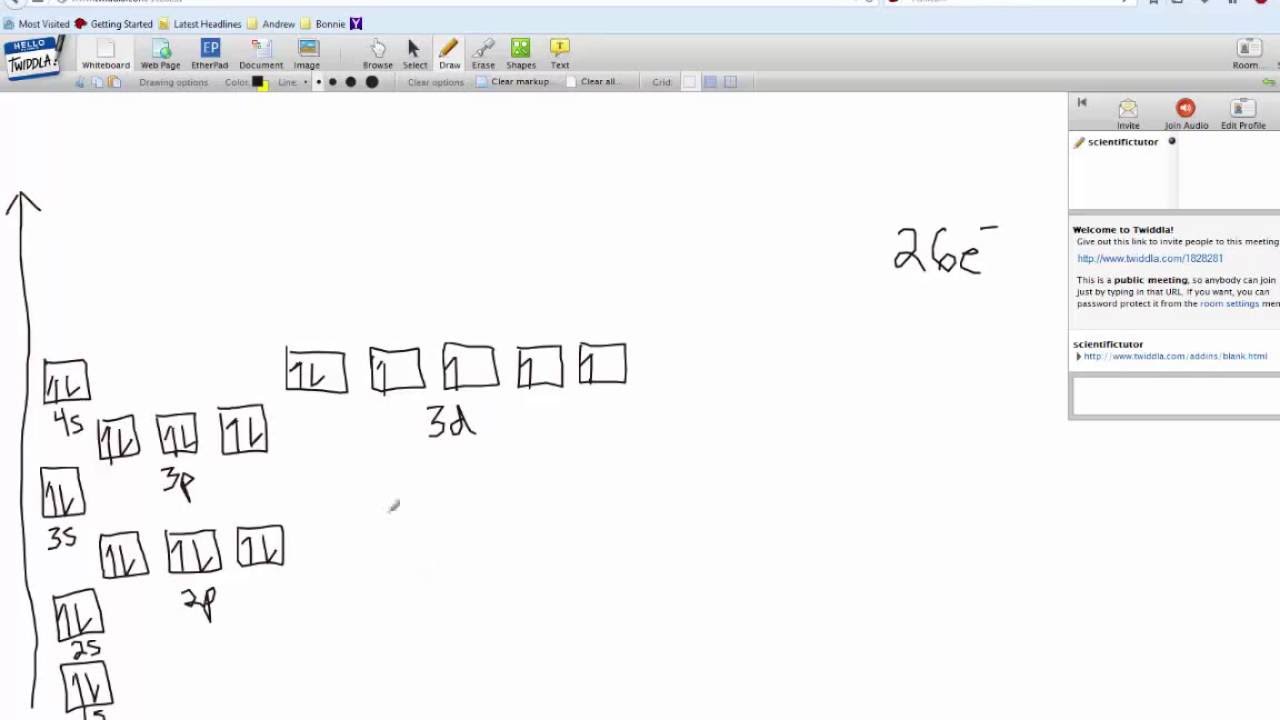
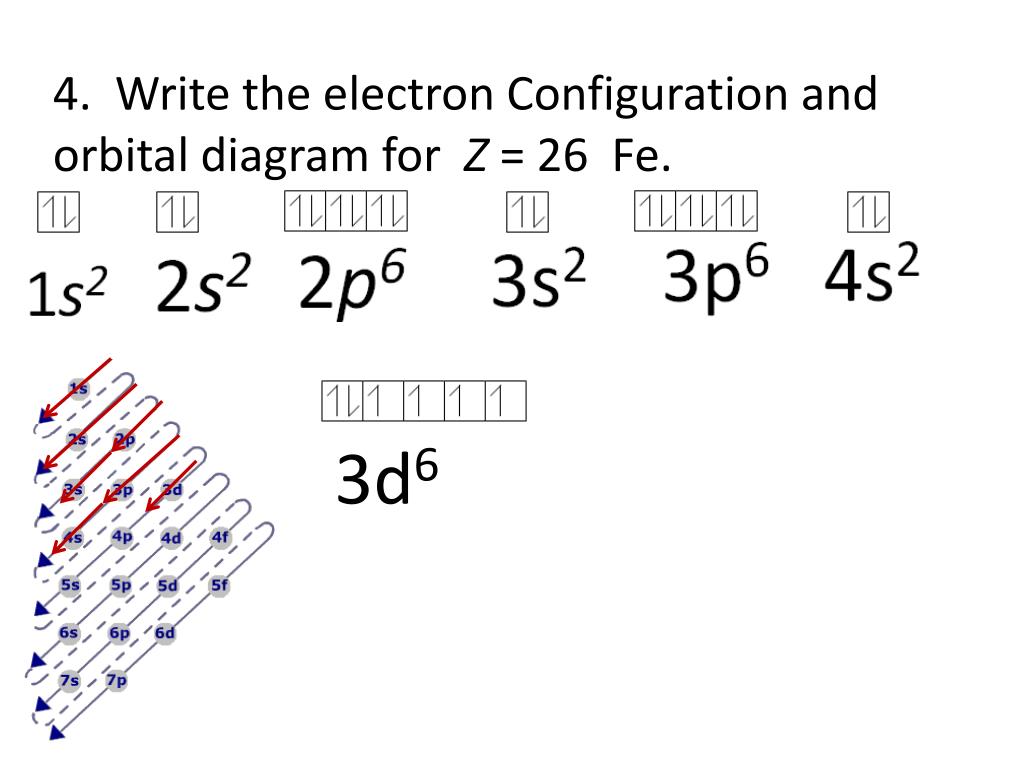
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of iron? - Answers Fe, or iron, has the atomic number of 26. Its full orbital diagram is 1s2 2s2 2p6 3s2 3p6 4s2 3d6.
Orbital diagram for fe. PDF The Electronic Structure of Ferrocene 1g molecular orbital is mainly ligand based with a slight admixture of the Fe 4s and 3dz2 orbitals. • Similarly the a 2u level has little if any metal character due to higher lying Fe 4p z orbitalwith which it is formallyable to combine. • The e 1g molecular orbital arises from the bonding combination of the ligand e 1g ... Solved 10. Write the electron configuration and ... - Chegg Chemistry questions and answers. 10. Write the electron configuration and draw the orbital diagram for iron (Fe), atomic number 26. Use the Noble core abbreviation. Electron Configuration of neutral iron: Orbital Diagram: Write the electron configuration and draw the orbital diagram for the chloride ion, (CI+), atomic number 17. Fe2+ Orbital Diagram - schematron.org 11.09.2018 11.09.2018 5 Comments on Fe2+ Orbital Diagram. For midterm question Q5C, why the electron configuration for Fe2+ is not [Ar]3d^5 4S^1? The outermost shell, in this case, is the 4s orbital. ... Iron is a chemical element with symbol Fe (from Latin: ferrum) and atomic number It is a metal in the first transition schematron.org is by ... F. what is the orbital diagram and the corresponding ... 26Fe has the configuration 1s22s22p63s23p63d64s2. A point to be noted : Note that the 4s orbitals are filled AFTER you add six electrons to the 3d orbitals. The 3d orbitals belong with the n=3 orbitals. So, you will have 2 electrons in the n=1 (1s) shell, 8 in the n=2 (2s and 2p) shell, 8+6 = 14 in the n=3 (3s, 3p, and 3d) shell,
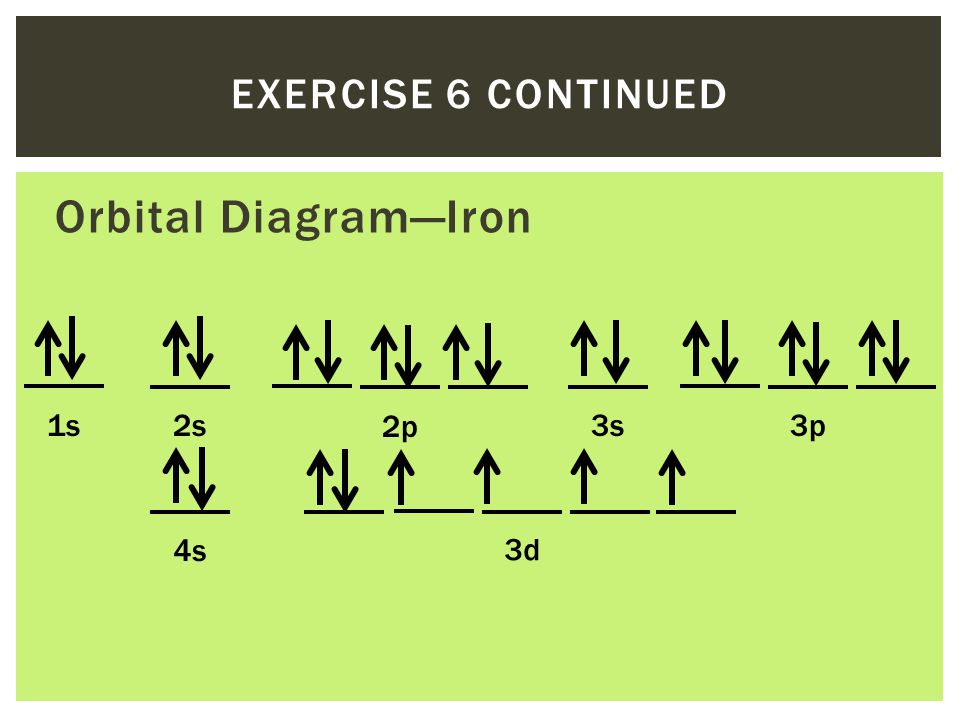
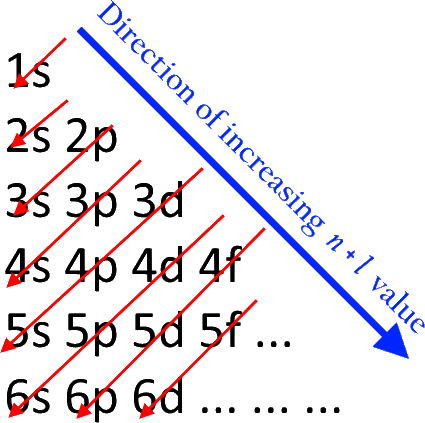
Schematic molecular orbital diagram for Fe III Cl 4 Ϫ ... Download scientific diagram | Schematic molecular orbital diagram for Fe III Cl 4 Ϫ . The Fermi level from publication: On the electronic structures of gaseous transition metal halide complexes ... PDF Orbital Diagrams - Mioy ORBITAL DIAGRAM Y ↿⇂ 1s ↿⇂ 2s ↿ ↿ ↿ 2p N Fill orbitals with "up" before "down" to maximize number of unpaired electrons. ↿⇂ 1s ↿⇂ 2s ↿⇂ ↿⇂ ↿⇂ 2p Na ↿⇂ 3s ↿⇂ 3s Fe ↿⇂ ↿⇂ ↿⇂ 3p ↿⇂ 4s ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 3d Let's only worry about the outermost orbitals for Fe. PDF Example: Constructing a only MO diagram for Iron ... Example: Constructing MOs for Titanium Tetraiso propoxide, Ti(OiPr) pp , 4 • The OiiPrSALCs are compridised of fill dfilled p d bit l th O t x an p y orbitals on e a oms. • Ti bonding AOs E: (3dz 2, 3dx2‐y ) T 2: (4p x , 4p y, 4p z) (3dxy, 3dxz, 3dyz) • The T 1 SALC is non‐bonding. • Significant overlap occurs between the E SALC 2and the e AOs on Ti (3dz2, 3dx2‐y ) How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Electron Configuration for Iron (Fe, Fe2+, and Fe3+) Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions) In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Iron go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. Iron(Fe) electron configuration and orbital diagram Iron (Fe) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. How do you determine the electron configuration of Fe ... The ground state electron configuration of Fe is: "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"3d"^6"4s"^2" For all but about 20 transition metals, the Aufbau diagram is a useful tool that helps to determine the ground state electron configuration of an element. Iron (Fe) is a transition metal that follows the Aufbau rule of the filling of atomic orbitals. 36 orbital diagram of fe - Diagram Online Source Atomic Orbital Diagram for Iron (Fe) Iron ion (Fe 2+,Fe 3+) electron configuration. Ground state electron configuration of iron (Fe) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. The electron configuration shows that the last shell of iron has two electrons and the d-orbital has a total of six electrons. In this case, the valence electrons of iron are ...
How to Draw Orbital Diagrams - YouTube Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
Iron (Fe) - ChemicalAid Iron (Fe) has an atomic mass of 26. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
Titanium(Ti) electron configuration and orbital diagram The Aufbau principle is that the electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital. These orbitals are named s, p, d, f. The electron holding capacity of these orbitals is s = 2, p = 6, d = 10 and f = 14.
40 orbital diagram of fe - Diagram For You Atomic Orbital Diagram for Iron(Fe) Iron ion(Fe 2+,Fe 3+) electron configuration. Ground state electron configuration of iron(Fe) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2.The electron configuration shows that the last shell of iron has two electrons and the d-orbital has a total of six electrons.
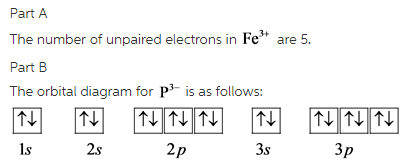
Orbital Diagram For Fe3+ - schematron.org Orbital Diagram For Fe3+ 06.02.2019 4 Comments There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals).
PDF Electron Configurations and Orbital Diagrams key Electron Configurations and Orbital Diagrams KEY Draw orbital diagrams for the following elements: 1. phosphorus ... 14. Fe +3 1s 2 2s 2 2p 6 3s 3p 6 3d 5 [Ar] 3d 5 15. Mn 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 [Ar] 3d 5 16. W+1 1s 2s 6 3s 3p 3d 10 4s 4p 4d 4f14 5s 5p 5d 4 6s 1 14 5d 4 6s 1 17. Mo +3
Qualitative valence molecular-orbital (MO) diagram of Fe ... Download scientific diagram | Qualitative valence molecular-orbital (MO) diagram of Fe(CO) 5 . Displayed is the subset of Fe(CO) 5 MOs which are derived from Fe 3d and CO 5r and 2p orbitals. For ...
What is the orbital diagram for Fe? - Answers What is the orbital diagram for Fe? - Answers Fe, or iron, has the atomic number of 26. diagram is 1s2 2s2 2p6 3s2 3p6 4s2 3d6. Home Study Guides Science Math and Arithmetic History Literature and...
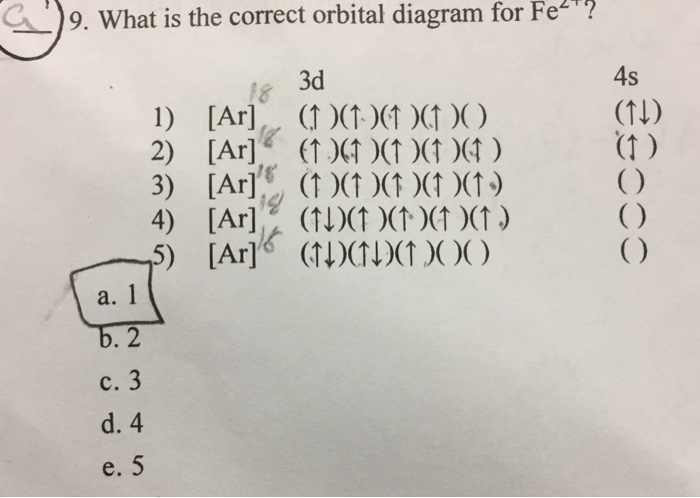
Solved 9. What is the abbreviated electron configuration ... Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: 9. What is the abbreviated electron configuration and orbital diagram for Fe? Is it paramagnetic or diamagnetic?
Orbital Diagram For Fe3+ Orbital Diagram For Fe3+ Atomic orbitals, electron configurations and the. Periodic Table. Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals).
Orbital diagram of iron? - Answers Fe, or iron, has the atomic number of 26. Its full orbital diagram is 1s2 2s2 2p6 3s2 3p6 4s2 3d6.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35:
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom






/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)










![Why is[FeF6]3- ion paramagnetic while [Fe(CN)6]4-ion ...](https://miro.medium.com/max/564/0*8MKpf7WYyO4EenzP.jpg)

0 Response to "40 orbital diagram for fe"
Post a Comment