39 Orbital Diagram For Beryllium
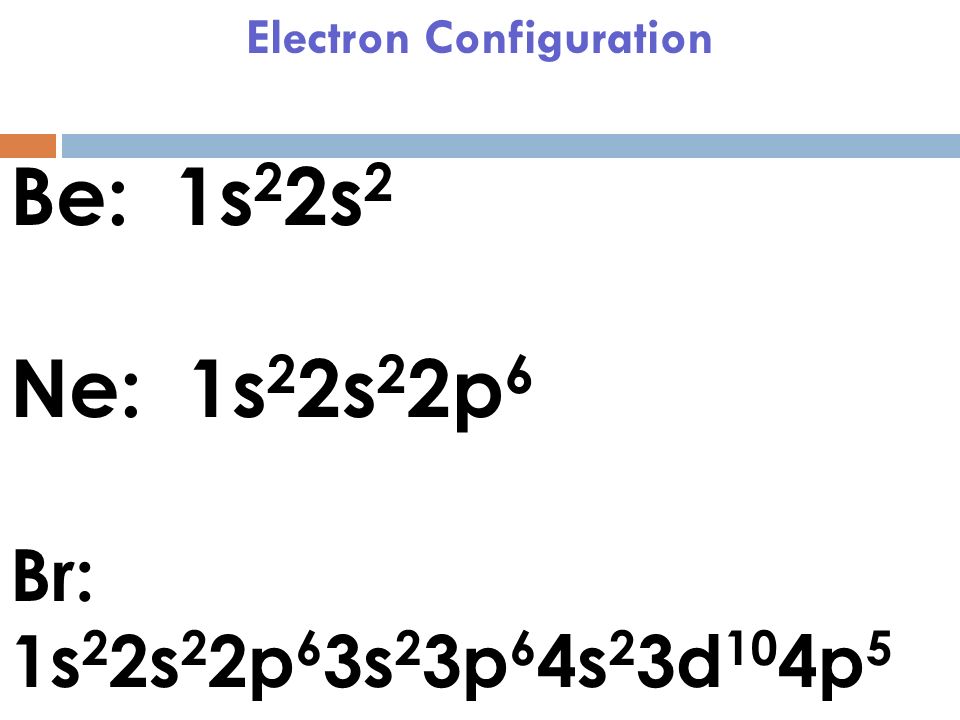
Orbital Diagram For Beryllium Nov 30, 2018 · Since the 2s orbital is completely filled, a new type of orbital must be of one more atom, carbon, with the aid of the color-coded diagrams. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of beryllium-9 (atomic number: 4), the most common. 6 Chemical Bonding 12.2.2018 · CHAPTER 6 REVIEW Chemical Bonding SECTION 3 SHORT ANSWER Answer the following questions in the space provided. 1. a The notation for sodium chloride, NaCl, stands for one (a) formula unit. (c) crystal. (b) molecule. (d) atom. 2. d In a crystal of an ionic compound, each cation is surrounded by a number of (a) molecules. (c) dipoles. (b) positive ions. (d) …
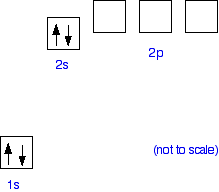
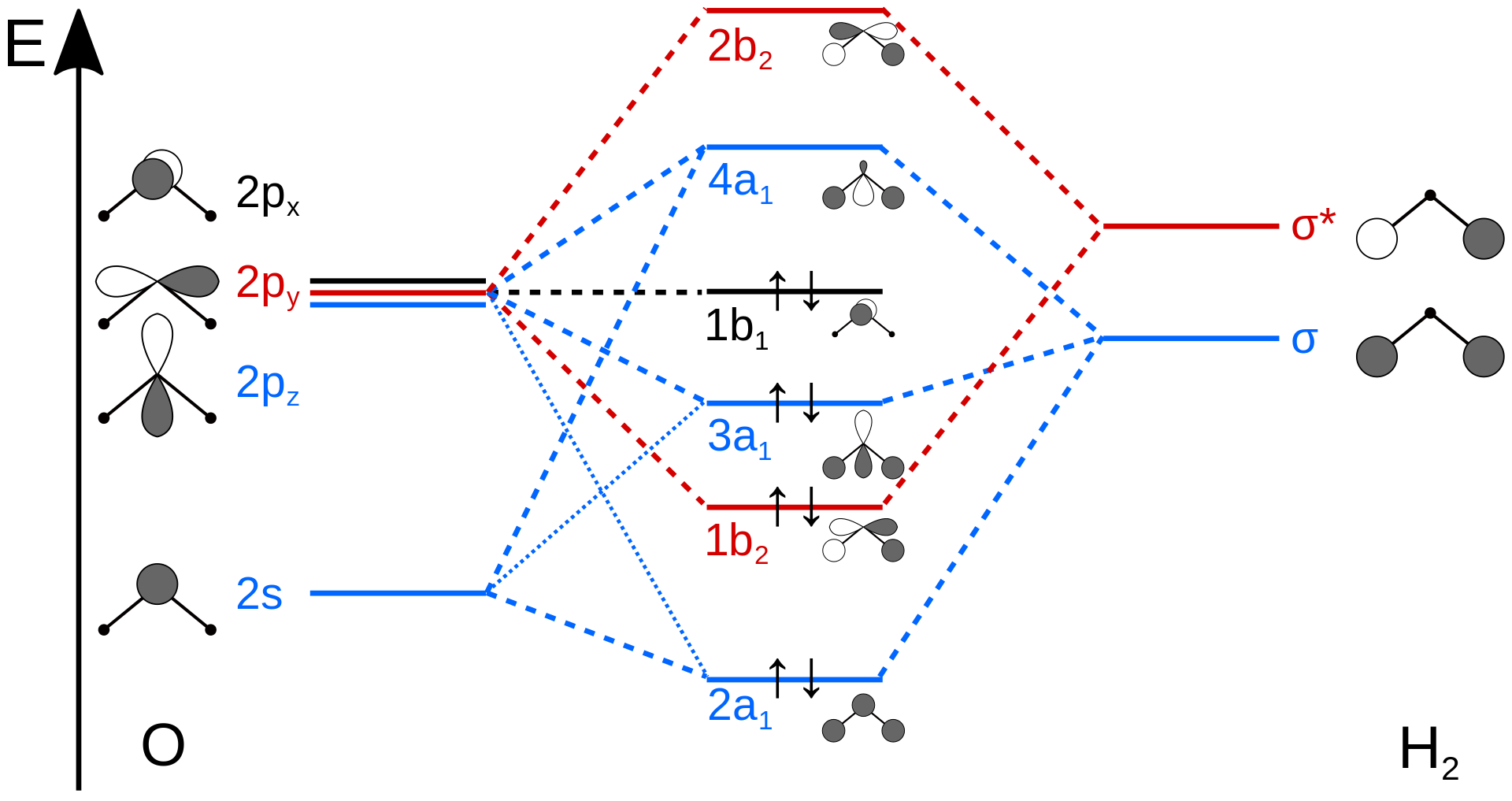
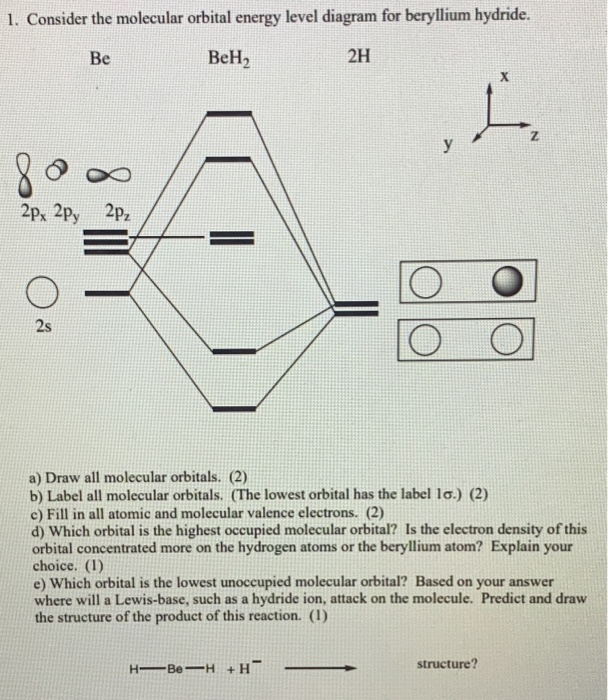
Beryllium Orbital Diagram - schematron.org Apr 16, 2019 · Beryllium Orbital Diagram. A quiz solution for Inorganic Chemistry in which students were prompted to draw the molecular orbital diagram for beryllium hydride. Beryllium is the fourth element with a total of 4 electrons. In writing the electron configuration for beryllium the first two electrons will go in the 1s orbital.
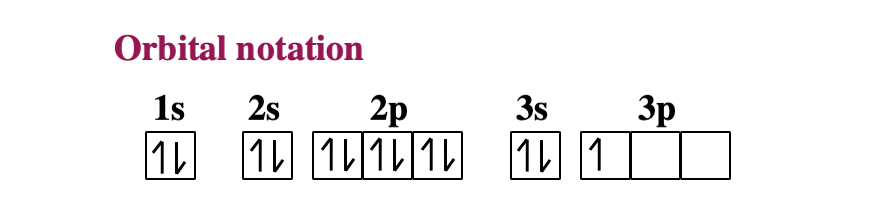
Orbital diagram for beryllium
Orbital energy diagram for beryllium? | Socratic Well, the atomic orbital (AO) ordering is quite normal and predictable. BERYLLIUM AO ENERGY ORDERING. Be 's ground-state electron configuration is the one ... Polarizability - Chemistry LibreTexts For Beryllium compared to the other alkaline earth metals: With water: All Group 2 metals except Be, react with water M(s) + 2H 2 O(l) → M 2+ (aq)+ 2OH-(aq) + H 2 (g) With oxygen (air): Be only reacts with air above 600 °C if it is finely powdered. The BeO that is formed is amphoteric (other Group 2 oxides are basic). 10 shocking facts about NASA's James Webb space telescope ... 22.12.2021 · James Webb Space Telescope’s primary mirror at NASA Goddard. The secondary mirror is the round mirror located at the end of the long booms, which are …
Orbital diagram for beryllium. BeH2 Lewis Structure, Molecular Geometry, Hybridization ... 2 päivää sitten · Now, the 2s orbital of the beryllium atom fuses with its 2p orbital and hence, form two sp hybrid orbitals of equivalent energy, which align themselves in a linear geometry. The sp hybrid orbital of the beryllium atom overlap with the 1s atomic orbital of the hydrogen atom, which is shown in the orbital diagram of the beryllium hydride molecule as follows: Beryllium Orbital Diagram Jan 21, 2019 · Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of beryllium-9 (atomic number: 4), the most common. Since the 2s orbital is completely filled, a new type of orbital must be of one more atom, carbon, with the aid of the color-coded diagrams. Magnesium(Mg) electron configuration and orbital diagram Orbital Diagram for Magnesium (Mg) Electron configuration of magnesium ion(Mg 2+) Ground state electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. After the electron configuration, the last shell of the magnesium atom has two electrons. In this case, the valency and valence electrons of magnesium are 2. Atomic Structure Chemistry Quiz - ThoughtCo 6.3.2017 · A beryllium atom has 4 protons, 5 neutrons, and 4 electrons. ... The principal quantum number is an indication of the size of an electron orbital. It has a positive integer value, but can never be 0. So, ... Chlorine's electron dot diagram is Cl surrounded by seven dots.
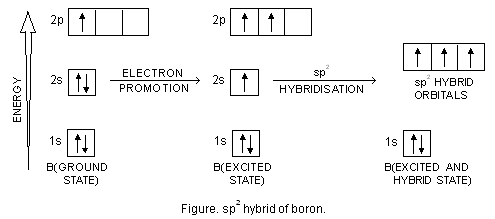
sp Hybridization | Introduction to Chemistry In sp hybridization, the s orbital overlaps with only one p orbital. Atoms that exhibit sp hybridization have sp orbitals that are linearly oriented; two sp orbitals will be at 180 degrees to each other.. Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. Some examples include the mercury atom in the linear … Chapter 2 structure of atom class 11 - SlideShare 30.6.2015 · Chapter 2 structure of atom class 11 1. Structure of Atom 1 GRADE 11 2. DISCOVERY OF AN ELECTRON An electron was discovered by cathode ray discharge tubes experiment. A cathode ray tube is made of glass containing two thin pieces of metal called electrodes, sealed in it. Beryllium(Be) electron configuration and orbital diagram Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit of the element is 2n2. For example, 1. n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2 electrons. 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8 electrons . 3. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. 4. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 42= 32 electrons. The atomic number is the number of electrons in tha... Beryllium (Be) - ChemicalAid Beryllium (Be) has an atomic mass of 4. ... 2 (alkaline earth metals or beryllium family) ... Be - Beryllium - Orbital Diagram - Electron Configuration ...
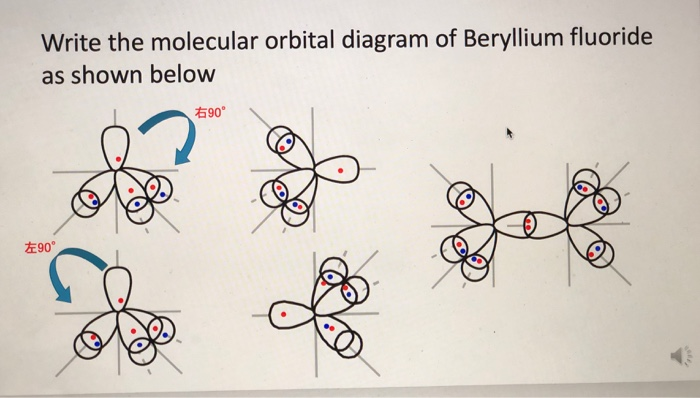
Quantum Number Questions and Answers | Study.com Quantum Number Questions and Answers. Get help with your Quantum number homework. Access the answers to hundreds of Quantum number questions that are explained in a way that's easy for you to ... Carbon Bohr Model - How to draw Bohr diagram for Carbon(C ... The Bohr Model of Carbon(C) has a nucleus that contains 6 neutrons and 6 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Carbon contains 4 electrons that also called valence electrons. Orbital Diagram For Beryllium - schematron.org Mar 21, 2019 · Beryllium is the fourth element with a total of 4 electrons. In writing the electron configuration for beryllium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the remaining 2 electrons for Be go in the 2s orbital. Dec 29, · An advanced molecular orbital diagram of BeH2 (beryllium hydride) for the ... How Can We Find A Beryllium Electron Configuration (Be) 4 Jan 2021 — Beryllium Electron Configuration: Beryllium is the fourth atomic number of the periodic table. The symbol of Beryllium is “Be” and it is a ...
10 shocking facts about NASA's James Webb space telescope ... 22.12.2021 · James Webb Space Telescope’s primary mirror at NASA Goddard. The secondary mirror is the round mirror located at the end of the long booms, which are …
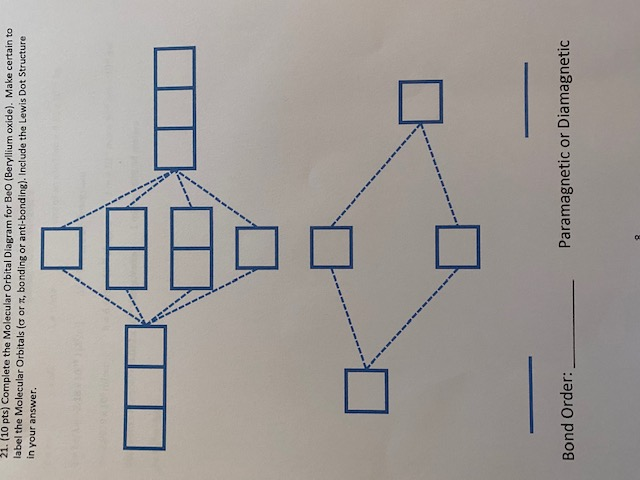
Polarizability - Chemistry LibreTexts For Beryllium compared to the other alkaline earth metals: With water: All Group 2 metals except Be, react with water M(s) + 2H 2 O(l) → M 2+ (aq)+ 2OH-(aq) + H 2 (g) With oxygen (air): Be only reacts with air above 600 °C if it is finely powdered. The BeO that is formed is amphoteric (other Group 2 oxides are basic).
Orbital energy diagram for beryllium? | Socratic Well, the atomic orbital (AO) ordering is quite normal and predictable. BERYLLIUM AO ENERGY ORDERING. Be 's ground-state electron configuration is the one ...


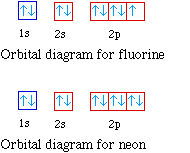

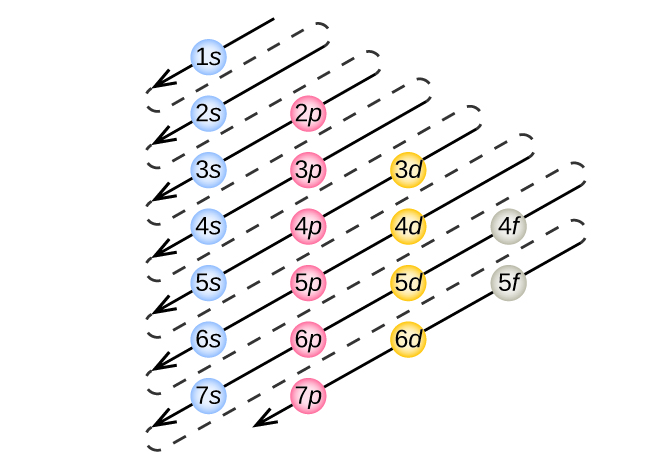




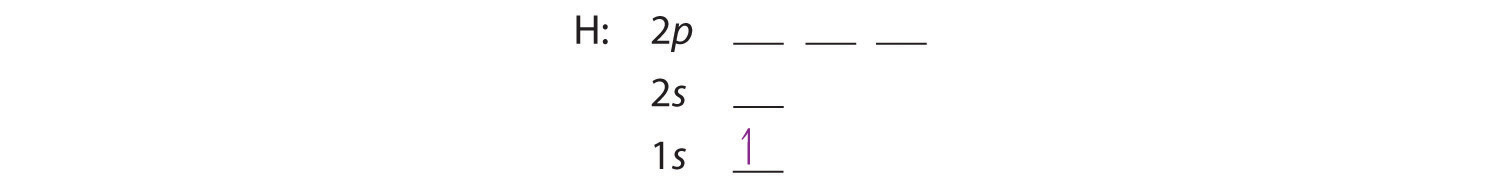
















![Solved Review Topics] the References to access important ...](https://media.cheggcdn.com/media%2Fb36%2Fb366a881-6786-4550-9679-2288f04044f5%2Fimage)
0 Response to "39 Orbital Diagram For Beryllium"
Post a Comment