39 lewis dot diagram for ph3
Phosphine PH3 Lewis Dot Structure - YouTube A video explanation of how to draw the Lewis Dot Structure for Phosphine, along with information about the compound including Formal Charges, Polarity, Hybri... Phosphine - Structure, Properties, Preparation, Uses and FAQ The diagram of Phosphine is studies based on the Lewis structure. Lewis structure is also called electron dot structures and is a picture to represent the lone pair of electrons and bonds with atoms or molecules. This can be grasped quickly using the following pointers: Phosphine lewis structure has 8 valence electrons.
PH3 Lewis structure, Molecular geometry, Hybridization ... Phosphine, PH3 is a chemical compound that is a colorless, flammable, and very toxic compound. Though pure PH3 is an odorless, technical sample of phosphine that smells like garlic or decaying fish because of the presence of substituted phosphine and diphosphane (P2H4). Phosphine gas is used to manage a wide range of insects in sealed … PH3 Lewis structure, Molecular geometry, Hybridization ...

Lewis dot diagram for ph3
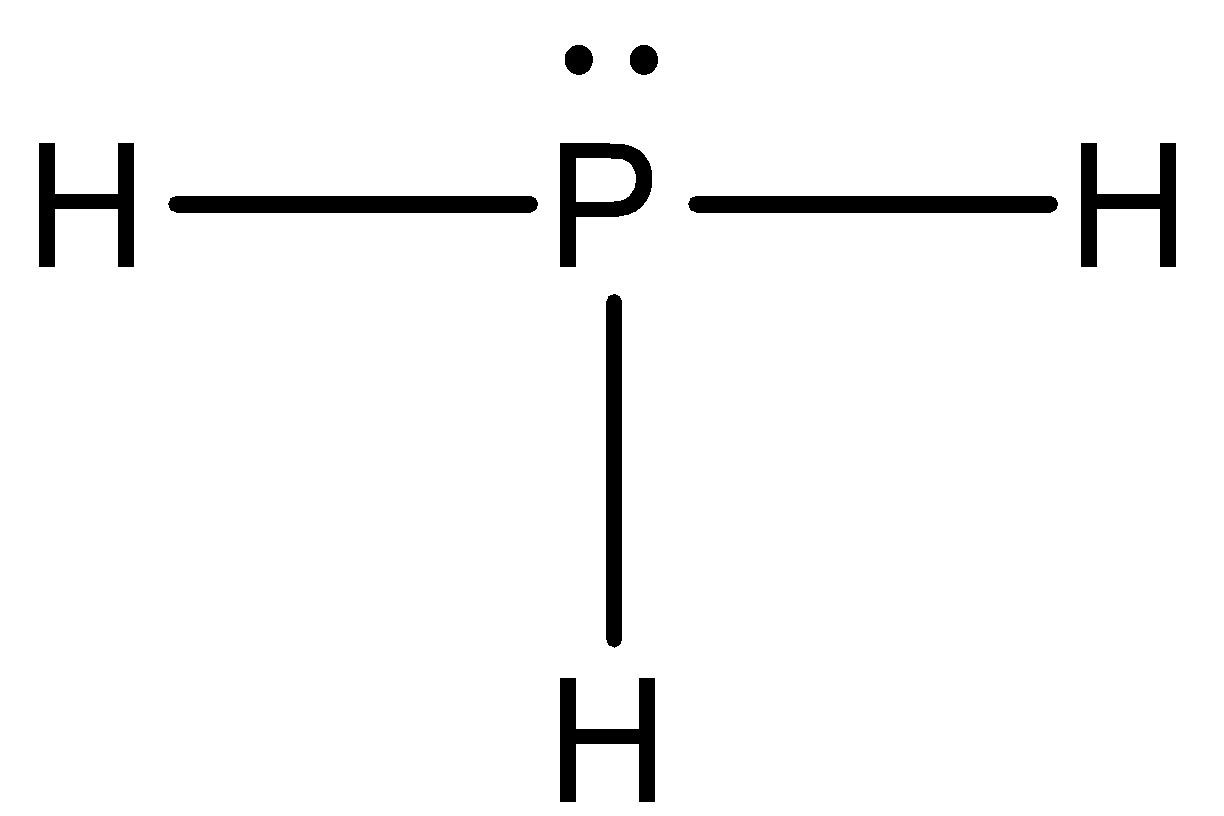
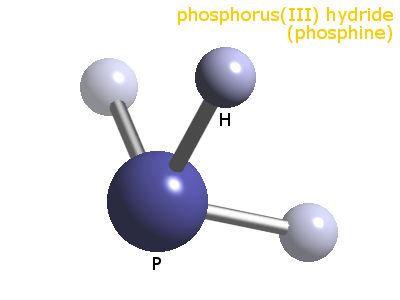
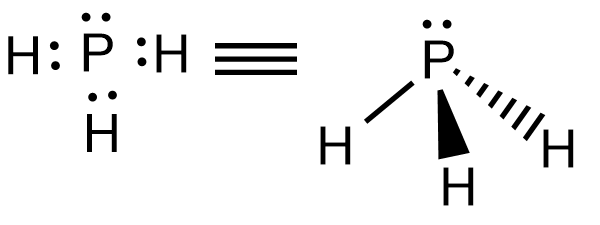
PH3 (Phosphine) Lewis Structure, Shape, Molecular Geometry In phosphine (PH 3) lewis structure, there are three sigma bonds and one lone-pair around phosphorous atom. No charges on phosphorous atom and hydrogen atoms. Shape of PH 3 is trigonal pyramidal. Molecular geometry around phosphorous atom is tetrahedral. Total valence electrons pairs around phosphorous atom is four. How can I draw the Lewis structure for PH3? | Socratic Chemistry Covalent Bonds Drawing Lewis Structures 1 Answer BRIAN M. Jul 16, 2014 Phosphosus has an electron configuration of [N e]2s22p3. Therefore, it has a dot digram in the red of two electrons on top and one electron on each side. Providing for three open bonding sites. Hydrogen has an electron configuration of 1s1. How to draw PH3 Lewis Structure? - Science Education and ... In the PH3 Lewis structure diagram, the Phosphorous atom can be the center atom of the molecule. As a result, central Phosphorous in the PH3 Lewis structure, with all three hydrogens arranged in the tetrahedral geometry. Add valence electrons around the hydrogen atom, as given in the figure.
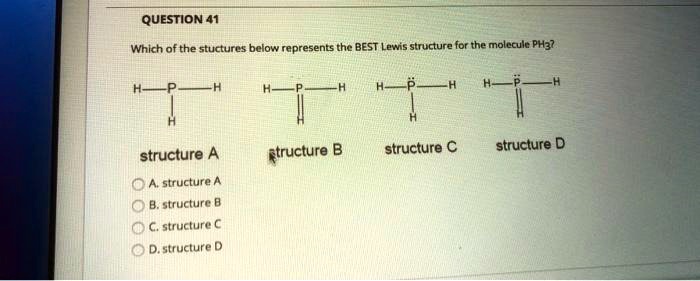
Lewis dot diagram for ph3. Lewis structure for PH3? - Answers What is the Lewis Structure of PH3? PH3 is phosphorus trihydride. dots above it. The three H's surround the P and are linked by a dash. Is PH3 a Lewis acid or base? It is a lewis base because of... Phosphorus trifluoride (PF3) lewis dot structure ... The molecular geometry or shape for PF3 is the trigonal pyramid. The electron geometry for PF3 is tetrahedral as it central has 4 regions of electron density. Lewis dot structure of PF3 contains 1 lone pair on the central atom (phosphorous) and 3 lone pairs on each outer atom (fluorine). Subscribe to Blog via Email Lewis Dot Structure Of Ph3 - sf4 lewis structure how to ... Lewis Dot Structure Of Ph3. Here are a number of highest rated Lewis Dot Structure Of Ph3 pictures on internet. We identified it from obedient source. Its submitted by supervision in the best field. We receive this kind of Lewis Dot Structure Of Ph3 graphic could possibly be the most trending topic taking into consideration we allocation it in ... Answered: Predict the shape of PH3 based on VSEPR… | bartleby Predict the shape of PH3 based on VSEPR theory. Show your work using Lewis dot structure. Central atom is in Bold. After drawing the Lewis structures answer the following. a) PH3 Central atom Number of bonds to the central atom Nonbonding pairs on the central atom Total bonds + nonbonding pairs Shape of the molecule.
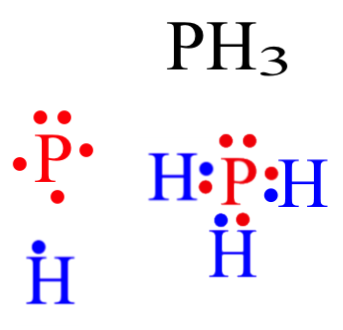
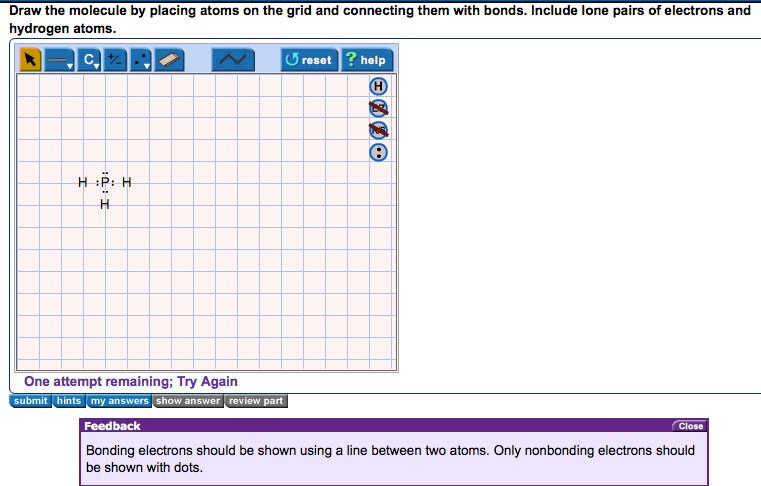
What Is The Lewis Dot Structure Of Ch2o? What geometry will this molecular structure have PH3? The PH3 molecule has a tetrahedral geometry shape because it contains three hydrogen atoms. There are three P-H bonds at the PH3 molecular geometry. After linking the three hydrogens and one lone pair of electrons in the tetrahedral form, it maintains the tetrahedral-like structure. What is ... How do you draw the Lewis structure for PH3 class 11 ... By Lewis dot structures we can visualize the bonding electrons and lone pair of electrons present in the molecule. Complete answer: - In the question it is given that to draw the Lewis dot structure of the phosphine. - We know that the atomic number of phosphorus is 15. - The electronic configuration of phosphorus is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3 . Draw the Lewis structure of PH3.To add lon... | Clutch Prep Problem: Draw the Lewis structure of PH3.To add lone pairs, click the button before clicking on the molecule.To add bonds connect atoms with a line .Draw the molecule by placing atoms on the grid and connecting them with bonds. Include lone pairs of electrons and hydrogen atoms.Use the following steps to draw Lewis structures from molecular formulas:1. PH3 Lewis Structure: How to Draw the Dot Structure for PH3 The Lewis structure for PH 3 is similar to NH 3. In the PH 3 Lewis structure (and all Lewis structures) hydrogen goes on the outside. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. In the Lewis structure for PH 3 there are a total of 8 valence electrons.
does ph3 follow the octet rule A.BH3 B.NH3 C.PH3 D.H2S E.All Of These Obey The Octet Rule. it is the compound which does not follow the octet rule for electron distribution c) SF4 4 dots on the I. Sulfur, phosphorus, silicon, and chlorine are common examples of elements that form an expanded octet. I believe a possible Lewis dot structure that does not obey the octet rule ... TOP ANSWER: Lewis Dot Structures Worksheet Lewis Dot ... For the compounds listed below, draw the correct Lewis Dot Structure. 11. SiCl4 12. OF2 13. CCl4 14. PF3 15. H2O 16. SO2 . 17. PH3 18. CO 19. BF3 20. CCL2O 21. CH3OH 22. I2 Drawing Lewis Dot Structures 1. Add up the total number of valence electrons in the molecule by totaling the valence What is the Lewis dot structure for PH3? - Answers The electron dot structure for PH3 is simple. Place a P atom in the center and single bond it to three separate hydrogen atoms. Place a lone pair on the P atom. Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
How to draw AsH3 Lewis Structure? - Science Education and ... Arsenic in the AsH3 Lewis structure, with all three hydrogens arranged in the tetrahedral geometry. Add valence electrons around the hydrogen atom, as given in the figure. Step-3: Lewis dot Structure for AsH3 generated from step-1 and step-2. Connect the exterior and core central atom of the AsH3 molecule with three single bonds (As-H).
PH3 Lewis Structure - How to Draw the Lewis ... - YouTube A step-by-step explanation of how to draw the PH3 Lewis Dot Structure (Phosphine).For the PH3 structure use the periodic table to find the total number of va...
SOLVED: Lewis Dot Structures Worksheet Lewis Dot ... For the compounds listed below, draw the correct Lewis Dot Structure. 11. SiCl4 12. OF2 13. CCl4 14. PF3 15. H2O 16. SO2 . 17. PH3 18. CO 19. BF3 20. CCL2O 21. CH3OH 22. I2 Drawing Lewis Dot Structures 1. Add up the total number of valence electrons in the molecule by totaling the valence
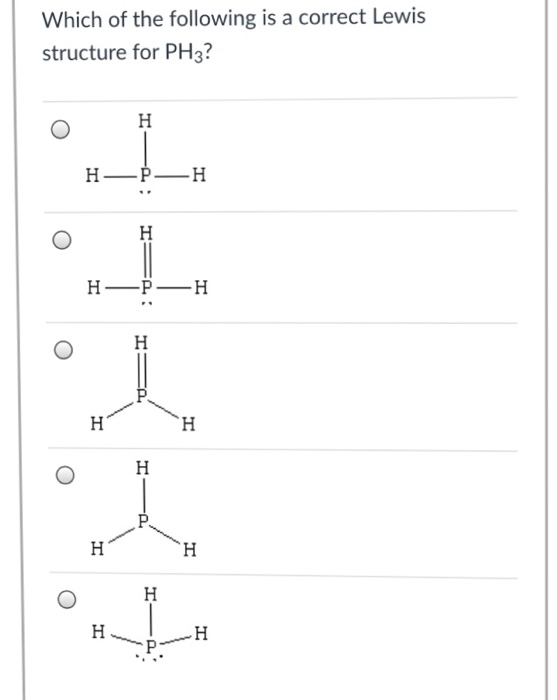
Solved Draw the Lewis structure of PH3. | Chegg.com Draw the Lewis structure of PH3. Question: Draw the Lewis structure of PH3. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Best Answer. This is the best answer based on feedback and ratings. 90% (10 ratings)..
PDF Lewis Dot Structures Pogil Key - Hudson City School District 9. Draw a Lewis dot diagram for the barium atom. 10. Draw the Lewis dot diagram for the silicon atom. I I, Draw the Lewis dot diagram for the iodine atom. 12. Draw the Lewis dot diagram for the xenon atom. 13. Hypothesize: Why are noble gases considered to be non-reactive? Your group will check your answers with the instructor before moving on.
PDF Lewis Dot Structures and VSEPR - Surry County Public ... • (3c) ·Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding.
How do you draw the lewis structure for ph3? | Socratic Here are the steps that I follow when drawing a Lewis structure. Decide which atom is the central atom in the structure. That will normally be the least electronegative atom (P). Draw a skeleton structure in which the other atoms are single-bonded to the central atom — a P atom with three H atoms attached to it. Draw a trial structure by putting electron pairs around every atom until each ...
PH3 Lewis Structure, Molecular Geometry, and Hybridization From the Lewis molecular structure of PH3, we have seen the phosphorous atom has five valence electrons. During the bonding process, Phosphorous is surrounded by three hydrogen atoms, and each is connected by a single bond. The two remaining electrons form a lone pair.
How to draw the lewis dot structures of covalent compound ... In the valence dot diagram or dot diagram or electron dot structure valence electron are shown using the dots, If we are drawing the electron dot diagram of covalent molecule the shared electron are shown as two dots or line between the atoms and the non bonding electrons are placed outside . To example : F2 molecule F .. F.
Lewis Structure for PH3 - UMD For the PH3 Lewis structure we first count the valence electrons for the PH3 molecule using the periodic table. Once we know how many valence electrons there are in PH3 we can distribute them around the central atom and attempt to fill the outer shells of each atom. The PH3 Lewis structure has 8 valence electrons.
BF3 Lewis structure, Molecular geometry, Hybridization ... BF 3 Lewis Structure. Gilbert N. Lewis gave a method to represent a molecule along with its chemical bonds and lone pair of electrons diagrammatically called Lewis or electron dot structure. The total number of lone and bound pairs of electrons in the atom is also shown in the Lewis structure.
How to draw PH3 Lewis Structure? - Science Education and ... In the PH3 Lewis structure diagram, the Phosphorous atom can be the center atom of the molecule. As a result, central Phosphorous in the PH3 Lewis structure, with all three hydrogens arranged in the tetrahedral geometry. Add valence electrons around the hydrogen atom, as given in the figure.
How can I draw the Lewis structure for PH3? | Socratic Chemistry Covalent Bonds Drawing Lewis Structures 1 Answer BRIAN M. Jul 16, 2014 Phosphosus has an electron configuration of [N e]2s22p3. Therefore, it has a dot digram in the red of two electrons on top and one electron on each side. Providing for three open bonding sites. Hydrogen has an electron configuration of 1s1.
PH3 (Phosphine) Lewis Structure, Shape, Molecular Geometry In phosphine (PH 3) lewis structure, there are three sigma bonds and one lone-pair around phosphorous atom. No charges on phosphorous atom and hydrogen atoms. Shape of PH 3 is trigonal pyramidal. Molecular geometry around phosphorous atom is tetrahedral. Total valence electrons pairs around phosphorous atom is four.





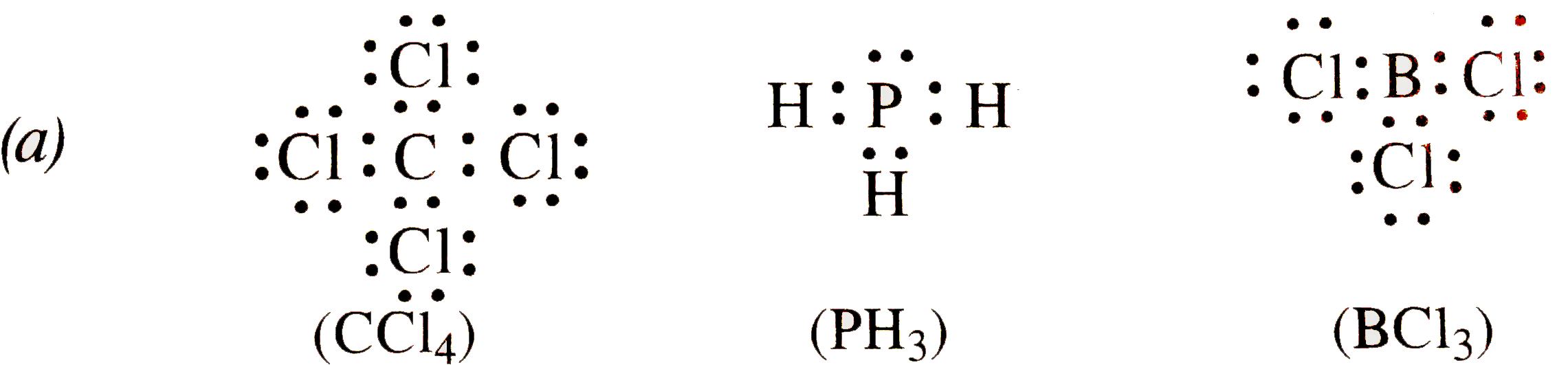






![Answered] Question 4.4 Draw the Lewis structures for the ...](https://hi-static.z-dn.net/files/d62/456674e06b9da2d91bbf3a122b777722.jpg)



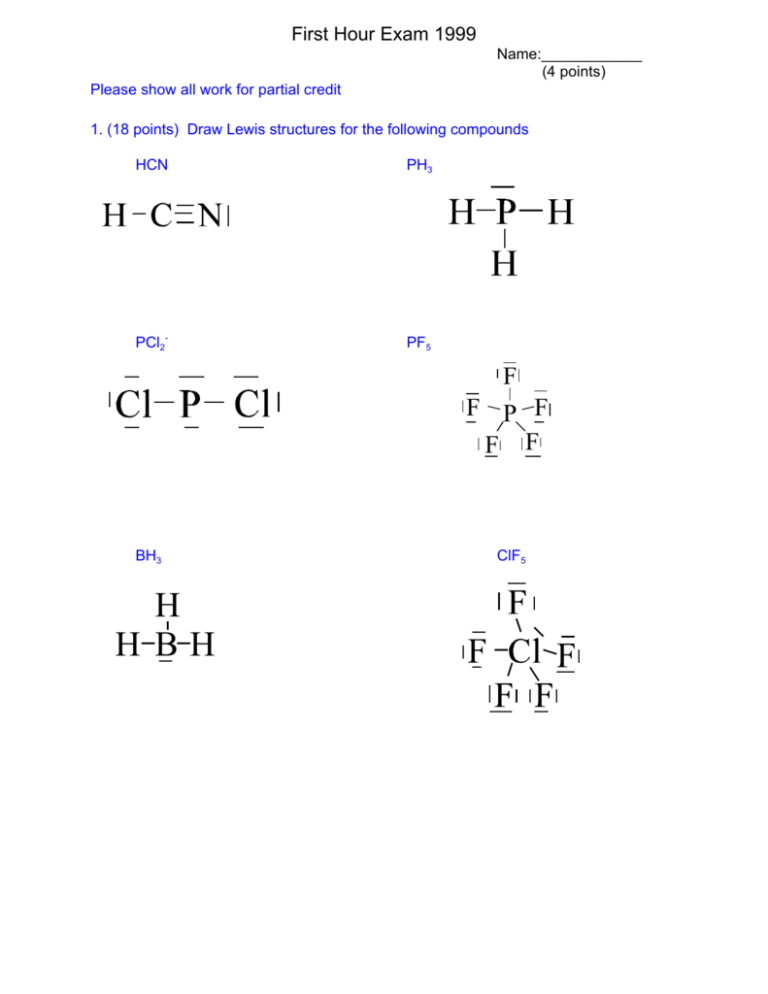



0 Response to "39 lewis dot diagram for ph3"
Post a Comment