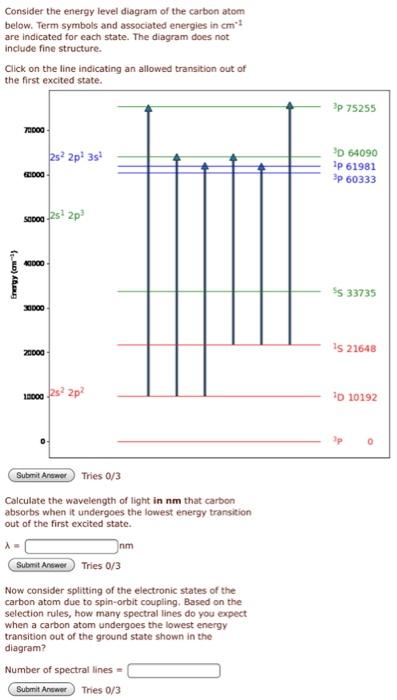
39 energy level diagram for carbon
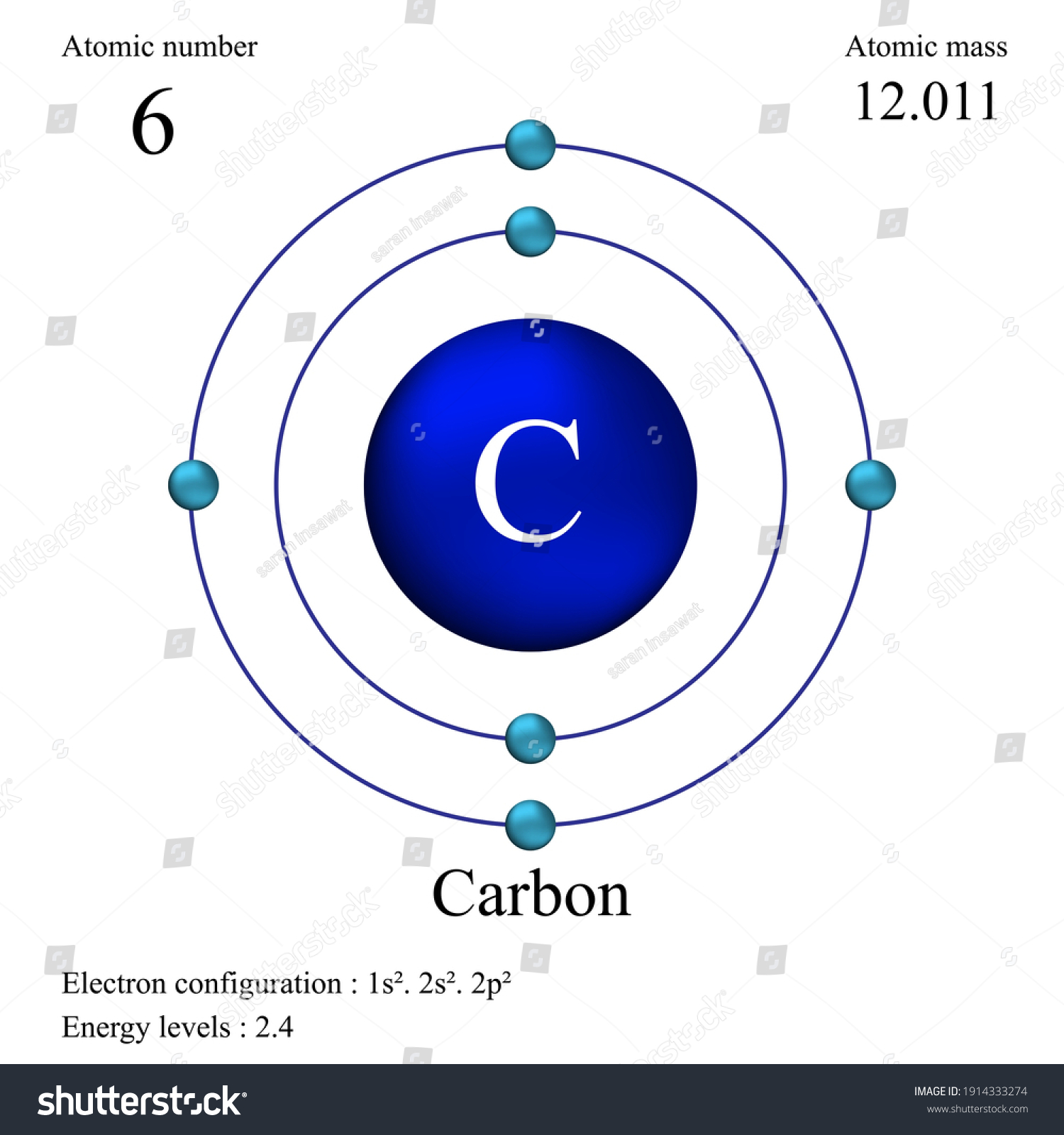
Carbon Atom - Edinformatics A better way to look at the carbon atom is by using an energy level graph shown at the right. Here we see carbon has six electrons represented by arrows (the direction of the arrow represents the electron spin) Two electrons are found in the 1s orbital close to the nucleus. Carbon energy level diagram - Big Chemical Encyclopedia Orbital energy level diagram for carbon oxysulphide using theoretical values (Clementi, 1962). The deeper levels are essentially localized atomic orbitals. The energy scale is in electron volts, expanded on the right to show the valence shell structure. [Pg.32] Fig. 2-16.—Energy-level diagram for the neutral carbon atom.
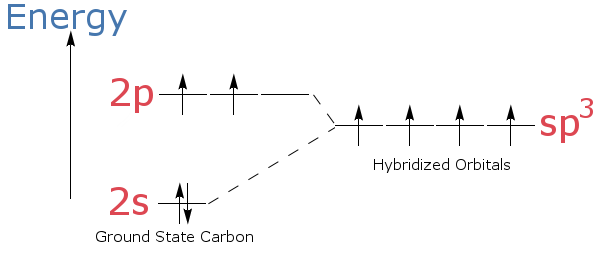
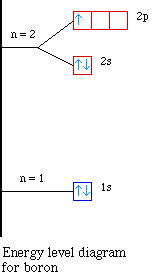
Carbon Energy Levels - Georgia State University The ground state electron configuration of carbon is 1s22s22p2. For excited states, the most typical situation is that five of the electrons maintain the configuration 1s22s22p1and a single electron is elevated. The states in the above diagram use the spectroscopic notationto characterize the state of that one electron.
Energy level diagram for carbon
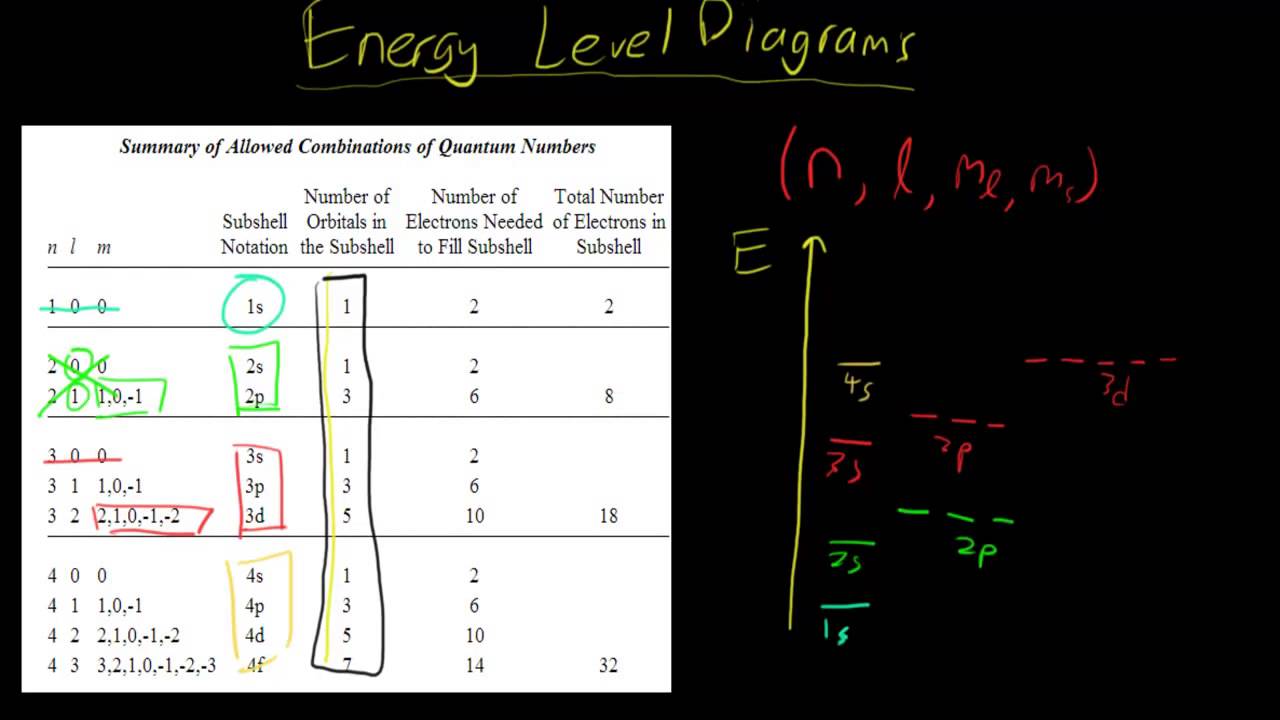
PDF Chapter 6 Nuclear Energy Levels Fig. 6-1. Energy level diagram of some of the excited states of the 12C nucleus. The angular momentum (J), parity (P), and isospin (T) quantum numbers of the states are indicated on the left using the notation J P. P and n respectively at the top of the diagram indicate the separation energies for a proton and a neutron. I was asked to fraw the energy level diagram for carbon ... Jul 7, 2009. If you are to draw the energy level for carbon (with no bonding) the electrons pair before moving to another orbital. For example, 6 C 12 would have an electron distribution in the ground state of. 1s2 2s2 2p2. I assume you are just starting chemistry; later on you will learn that one of the 2s2 electrons can be promoted to the ... PDF Energy Level Diagrams - Ms. Scott's Site The period # tells us how many energy levels an atom has. Example: Carbon is in the second row of the periodic table thus it is in period 2 and has 2 energy levels. Rules for Energy Level Diagrams for Atoms: 1. # protons = # electrons = atomic # 2. The maximum number of electrons in each energy level equals the number of atoms in each period (2,
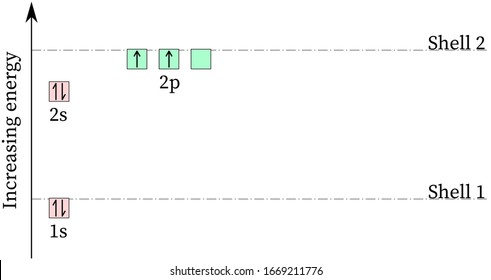
Energy level diagram for carbon. Carbon(C) electron configuration and orbital diagram Carbon (C) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. PDF How to Draw Bohr Diagrams period have one energy level. •Elements in the 2nd period have two energy levels, and so on. . Bohr Diagrams 1) Draw a nucleus with the number of protons and neutrons inside. 2) Carbon is in the 2nd period, so it has two energy levels, or shells. 3) Draw the shells around the nucleus. 6p 6n. Bohr Diagrams 1) Add the electrons ... energy level diagram of c2 - Brainly.in The Energy Level Diagram is used by the chemists along with a notation of electron configuration in order to represent the energy level, orbital, and sub-shell that is occupied by the electrons in a particular atom. In C2, the ground state electron configuration of carbon is 1s2 2s2 2p2. For excited states, the most typical situation is that ... Co2 Laser || Part - 2 || Working and Energy Level Diagram ... LINK OF " CO2 LASER : PART - 1 ( PRINCIPLE & CONSTRICTION " VIDEO ***** OF " Nd :...
How to Draw the Bohr-Rutherford Diagram of Carbon - YouTube Carbon has 2 electrons in its first shell and 4 in its second shell.Check me out: Solved Page 10. (16 points) Atomic Term Symbols. The ... The complete energy level diagram of the carbon atom, including fine structure, is shown on the next page. (a) (4 points) For the ground state electron configuration, draw the microstate for electrons in the 2p orbitals with the lowest energy. Identify L, S, and J, showing your work. Fill in the appropriate term. Energy Level Diagram - Different Energy Shells Around the ... Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy level is as follows: s ... Carbon Monoxide Molecular Orbital Diagram Explanation There are 4 electrons in the outer shell of carbon and 6.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
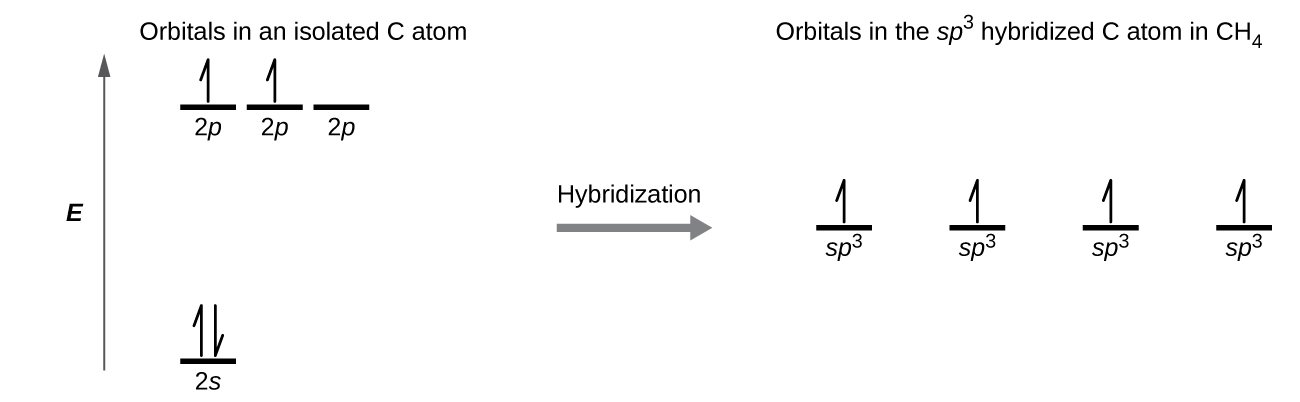
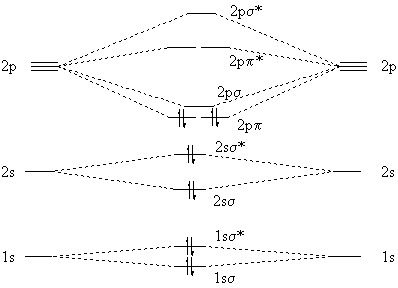
PDF Covalent Bonds & Shapes of Molecules Energy-level diagram for carbon (atomic number 6) Energy. 5 • In the ground state of carbon, electrons are placed in accordance with the quantum chemistry principles (aufbau, Hund's rule, Pauli exclusion principle, etc.) that dictate the lowest energy form of carbon. Co2+ Orbital Diagram - schematron.org • Carbon Dioxide • Ammonia • Boron Trifluoride • Methane • Ethylene(Ethene) • Symmetry of Orbital Interactions. Metal reaction mechanisms • Polyhedral point groups Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. 3:05 (Triple only) draw and explain energy level diagrams ... The symbol ΔH is used to represent the change in heat (or enthalpy change) of a reaction. ΔH is measured in kJ/mol (kilojoules per mole). The change in heat (enthalpy change) can be represented on an energy level diagram. ΔH must also labelled. In an exothermic reaction, the reactants have more energy than Energy Levels, Orbitals, Sub-Levels and Shells - vigglegiggle Every energy level contains one S-Orbital. An S-Orbital in the first energy level is a 1s orbital. An S-Orbital in the second energy level is a 2s orbital etc. P-Orbitals: There are shaped like a 3D figure of eight. They exist in groups of three. Every energy level except the first level contains three P-Orbitals.
Energy level - Wikipedia Energy level diagrams. There are various types of energy level diagrams for bonds between atoms in a molecule. Examples Molecular orbital diagrams, Jablonski diagrams, and Franck-Condon diagrams. Energy level transitions. An increase in energy level from E 1 to E 2 resulting from absorption of a photon ...
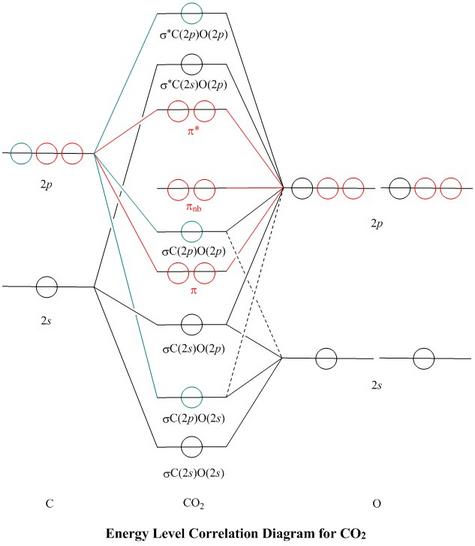
Molecular Orbitals for Carbon Monoxide The HOMO of carbon monoxide is σC(2p)O(2p) because the antibonding contribution from sp mixing pushes it above the π-bonding orbitals in energy Its main components are C 2 s and C 2 p z , so it is strongly polarised towards carbon, and will bond to σ -acceptor species through carbon, providing that the CO ligand is also acting as a π ...
PDF Energy Level Diagrams - Ms. Scott's Site The period # tells us how many energy levels an atom has. Example: Carbon is in the second row of the periodic table thus it is in period 2 and has 2 energy levels. Rules for Energy Level Diagrams for Atoms: 1. # protons = # electrons = atomic # 2. The maximum number of electrons in each energy level equals the number of atoms in each period (2,
I was asked to fraw the energy level diagram for carbon ... Jul 7, 2009. If you are to draw the energy level for carbon (with no bonding) the electrons pair before moving to another orbital. For example, 6 C 12 would have an electron distribution in the ground state of. 1s2 2s2 2p2. I assume you are just starting chemistry; later on you will learn that one of the 2s2 electrons can be promoted to the ...
PDF Chapter 6 Nuclear Energy Levels Fig. 6-1. Energy level diagram of some of the excited states of the 12C nucleus. The angular momentum (J), parity (P), and isospin (T) quantum numbers of the states are indicated on the left using the notation J P. P and n respectively at the top of the diagram indicate the separation energies for a proton and a neutron.
























0 Response to "39 energy level diagram for carbon"
Post a Comment