36 lewis dot diagram for h2s
Scientists Claim Hydrogen Sulfide To Be Beneficial For Health Scientists Have Found That Hydrogen Sulfide A Chemical Which Stin Chemistry Fun Facts Scientist . Carbon Monoxide Co Lewis Structure Chemistry Worksheets Lewis Carbon Monoxide . Glow In The Dark Lewis Dot Diagrams Senior Chemistry Quimica Organica Ligacao Quimica Ensino De Quimica Hydrogen Sulfide Lewis Dot Structure For H2s. Here are a number of highest rated Hydrogen Sulfide Lewis Dot Structure For H2s pictures upon internet. We identified it from obedient source. Its submitted by meting out in the best field.
Recently we began a chapter in Chemistry where we had to find the Lewis Dot Structures of Molecules (I.e. using valence electrons, adding in bonds, lone pairs, etc.) and I simply cannot get it. The process and sequence to get to the finished product is confusing, which is unfamiliar to me, as I am someone who typically very easily grasps concepts. I would very much appreciate anyone who could shed some light on their ways of thinking, easier way to go about it, or other tips, as my teacher has e...
Lewis dot diagram for h2s
Hydrogen Sulfide (H2S) Lewis Structure. Lewis structure of Hydrogen sulfide (H 2 S) contains two S-H single bonds around sulfur atom. Also, there are two lone pairs around sulfur atom. Concept of number of total valence electrons of sulfur and hydrogen atoms are used to draw lewis structure of H 2 S. Each step of drawing lewis structure of H 2 S is explained in detail in this tutorial. lewis dot diagram for sodium hypochlorite Draw the Lewis structures for the following molecules and ions: H2S, SiCl4, BeF2, , HCOOH. Answer. 1) H2S H has 1 electron in its valence shell & sulphur has 6 electron its valence shell. A planar structure will be formed . 2) Si has 4 electrons its valence shell & Cl need only 1 electron to complete its octet, therefore 4 Cl will make 4 bonds ...
Lewis dot diagram for h2s. This question has multiple correct options. Hard. View solution. >. Which statement is true about the most stable Lewis structure for C S 2 ? Medium. View solution. >. Draw an electron dot structure of the ethene molecule. Complete answer: The Lewis structure of H 2 S is very similar to H 2 O. Hydrogen is in Group 1 and therefore has only one valence electron. Hydrogen atoms only need 2 valence electrons to have a full outer shell. The Lewis structure for H 2 S has a total of 8 valence electrons. Hydrogen has 1 valence electron, but we have two Hydrogens here. To sketch the H2Se Lewis structure by following these instructions: Step-1: H2Se Lewis dot Structure by counting valence electrons on the selenium atom. Step-2: Lewis Structure of H2Se for counting valence electrons around the terminal hydrogen atoms. Step-3: Lewis dot Structure for H2Se generated from step-1 and step-2. Hello Everyone. For sale are the following items. Prices include shipping. PayPal or Venmo. No notes. Under 20 flair requires F&F or Venmo. Items will ship tomorrow. ~~Aimpoint H2 on a low mount (please note the little tool on the H2 lost a little nubbin)- $500~~ SOLD Modlite PLHV2 with surefire clicky cap. 2 x 18650 batteries, charger and USB - $315 Arisaka E2XTL with surefire clicky cap. One 18650 battery. - $220 BCM MCMR-9 M-Lok Handguard with barrel nut and original mounting i...
Lewis’s structure of SH2 is really helpful to determine its electron geometry, molecular shape, number of shared pair, and lone pair electrons. Follow these steps to draw the lewis dot structure for H2S. Step 1: In the first step, determine the total valence electron present in H2S. As we know hydrogen only has one valence electron in its last shell and Sulfur belongs to the 16th group in the periodic table so it contains 6 electrons in its last shell. Shouldn't oxygen have two bonds? or does the negative outside the brackets signify that both oxygen and hydrogen gain that one electron? Am I thinking about this properly?! I am having trouble with a question about a lewis dot diagram. If phosphorus and bromine atoms formed a compound, what would the lewis dot diagram and chemical formula be? Would the chemical formula be: PBr? (I couldn't find the formula anywhere on the internet to confirm if the formula is simply PBr). If it is not simply PBr, why or what makes it something else? If it is just PBr, would the lewis diagram be a single bond? \-I think I did it correctly, just wanting to make sure. H2s Lewis Dot Diagram. Here are a number of highest rated H2s Lewis Dot Diagram pictures on internet. We identified it from reliable source. Its submitted by admin in the best field. We recognize this nice of H2s Lewis Dot Diagram graphic could possibly be the most trending subject as soon as we share it in google plus or facebook.
of two electrons is shown by dots[ ].In H2S Lewis structure,there are two single bonds from sulphur to hydrogens.Besides,the atom sulphur has two lone pairs.Or,In H2S Lewis structure, the atom sulfur follows the octet rule and the hydrogen atoms follow the duet rule.So,H2S molecule follows the octet rule.In H2S Lewis structure,we get four Lewis dot Structure of H2S The chemistry graduates often encounter the hydrogen sulfide molecule due to the wide range of industrial applications. If we look into the formation of hydrogen sulfide molecules only two bonds of. If your student asks about the H2S Lewis Structure both hydrogen sulfide gas is colourless gas smell like rotten eggs ... (15) Lewis Diagrams Obj. 15. From the name of a molecular chemical, determine the Lewis (electron dot) diagram for it.. In these cases, you know that you are dealing with nonmetal atoms bonded together with covalent bonding and that (some of) the valence electrons of the atoms are shared between the atoms. The Lewis Structure of Hydrogen Sulfide is easy to draw and understand. In this compound, both the hydrogen atoms require one electron to make the covalent bond with Sulfur. The Lewis structure of H2S is similar to H 2 S. Sulfur needs eight electrons to fulfill the requirements for Octet Rule. But Hydrogen only requires a single electron to ...
Chemistry questions and answers. Consider the Lewis dot structure for each of the following and match it with the total number of non-bonded electrons in the molecule. (note the number in the formula are considered subscripts) CF4 24 < PF3 6 H2S 4 < v.
To draw the lewis dot structure of H₂S, we have to find out the valence electrons of sulfur and hydrogen first.We express valence electrons as dots in lewis dot structure. To get the valence electrons of sulfur,we need to look at the electronic configuration of sulfur. S (16)=1s²2s²2p⁶3s²3p⁴. More ›.
A step-by-step explanation of how to write the Lewis Dot Structure for H2S (Dihydrogen Sulfide).The H2S Lewis structure is similar to the structure for water...
Could some one please explain to me why [this](http://www.wolframalpha.com/input/?i=Thiocyanate&a=*C.Thiocyanate-_*ChemicalIntermediate-) sulfur has a negative charge? Thank You.
We are asked to draw the Lewis structure for H2S H 2 S and determine: the number of bonds and non-bonding pairs around the central atom; the shape of the ...
Hello! Can someone please explain to me how to draw the Lewis diagrams for transition metals? I understand how to find the valence electrons based on the electron configuration. For elements 27+, they begin having more than 8 valence? Please help I am confused!
This is the MO diagram of H2S. The left-hand side will contain the atomic orbitals of sulfur i.e 3s2 3px2 3py1 3pz1. And on the right-hand side, there will be atomic orbitals of hydrogen. 8 valence electrons are filled in the MO orbitals. There are two non-bonding orbitals present as well.
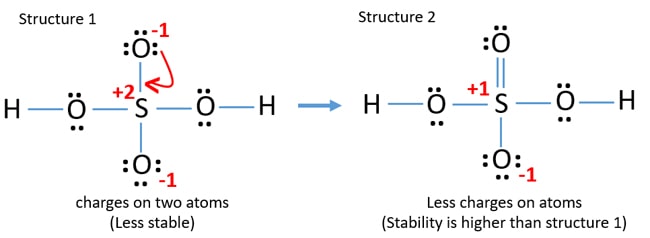
The Lewis dot structure has the S in the center. There are three O's surrounding the S. The O to the left and below the S have single bonds. While the O to the right has a double bond.
In the H2S Lewis structure diagram, the sulfur atom can be the center atom of the molecule. As a result, central sulfur in the H2S Lewis structure, with all two hydrogen atoms arranged in a tetrahedral geometry. Add valence electron around the hydrogen atom, as given in the figure. Step-3: Lewis dot Structure for H2S generated from step-1 and step-2
Click here👆to get an answer to your question ️ Draw the Lewis structures for the following molecules and ions : H2S, SiCl4, BeF2, CO^2 - 3 and HCOOH
What is the Lewis dot structure for H2S? The Lewis structure for H2S features a central sulfur atom, written as just the letter S, single-bonded to two hydrogen atoms, each represented by the letter H. There are four dots representing two lone pairs of electrons drawn above or below the sulfur atom. What is the name of the acid H2Se?
2. Draw the Lewis dot structures for each of the following molecules: a. H 2 S c. SO 3 b. CH 2 Br 2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH 4 + c. PO 4 -3 b. NO 3 - d. CO 3 2- 4. For the following molecules or ions (where the central atom is underlined): i. Draw the Electron dot structure. ii.
Drawing the Lewis Structure for H 2 S Viewing Notes: The Lewis structure for H 2 S is very similar to H 2 O. Hydrogen is in Group 1 and therefore has only one valence electron. But since you have two hydrogens you need to multiply by two. Hydrogen atoms only need 2 valence electrons to have a full outer shell.
What Is The Lewis Dot Structure For H2S. Hydrogen Sulfide is a common chemical compound that is advantageous for assessing inorganic link of steel ions. It has actually the chemistry formula the H2S. The molecule has actually two Hydrogen atoms and a single Sulfur atom. H2S is likewise a precursor for elemental Sulfur.
The MgBr2 molecule has one central magnesium atom and two bromine atoms. Then the total outermost valence shell electrons can be calculated as follows. ∴ Total outermost valence shell electrons available for MgBr2 Lewis structure ( dot structure) = 2 +2*7= 16 valence electrons in MgBr2. calculation of total valence electron of MgBr2 molecule.
Write the Lewis Electron Dot Structure Representation for the following: a) H2S b) CCl4 e) CO2 1) H2O 0 02 m) Br2 c) Cl2 3) CS2 n) SIO2 9) N2 d) CH4 k) F2 h) NH3 1) 12 . This problem has been solved! See the answer See the answer See the answer done loading.
Sup dudes. Trying this again. I got my green eotech, now I want a green variant of the MRO. https://imgur.com/a/288Mepc Original owner blacked out the logo with Aluma black, and honestly looks almost exactly like the T2 does from factory. Optic works flawlessly and the glass is clear. It’s sitting on an ADM lower 1/3 mount. I also have the Aimpoint tool, but no OEM box. I only want to swap to green because of my color blindness. TV-$700 Not looking for anything else at this time. Please don’...
Hi, I am working on a project in which we need to draw the Lewis Dot Structure for five binary molecular compounds. Although (I think) I understand how to do these with normal elements, I am confused on how to do it with compounds. The teacher has allowed us to search these up (it is one small part of the project), but I am unable to find or understand what comes up. ​ Examples: Triphosphorus pentanitride (P3N5) Searching up brings up images that contain more than 3/5 of each el...
Mar 21, 2020 · The Lewis Dot Structure for H2S. Created by MakeTheBrainHappy. This is the Lewis Dot Structure for H2S (hydrogen sulfide). The rules for drawing lewis structures permit the replacement of the bond lines with two electrons. As we have previously discussed, hydrogen only requires two electrons to fill up its valence shell because it only has a 1s shell as the very first element in the periodic table.
If anyone can give me an explanation or help me understand how it will look.
If you have a link or image of one you can send to me that would be appreciated!
Based on logic, I would say that the answer is CO2 as it is unlikely that hydrogen would be lost other than in acid base reactions with water (which I don't think would produce H2 gas), and if carbon monoxide was released I think we would have a lot of problems. How could I justify the formation of CO2 using lewis structures? Is the question maybe looking for resonance models of HCO3- to show that it is acidic enough to be deprotonated?
This is the Lewis Dot Structure for H2O. You could alternatively also draw the structure by including two dots for every bond. While oxygen's octet seems to have been filled, hydrogen only has two electrons for its valence shell.
Answer (1 of 2): Lewis Structure for H2S (Dihydrogen Sulfide)||Lewis Dot Structure for H2S Hello,today I am going to draw the lewis structure for H2S in just four steps. Step-1: To draw the lewis dot structure of H₂S, we have to find out the valence electrons of sulfur and hydrogen first.We ex...
Draw the Lewis structures for the following molecules and ions: H2S, SiCl4, BeF2, , HCOOH. Answer. 1) H2S H has 1 electron in its valence shell & sulphur has 6 electron its valence shell. A planar structure will be formed . 2) Si has 4 electrons its valence shell & Cl need only 1 electron to complete its octet, therefore 4 Cl will make 4 bonds ...
lewis dot diagram for sodium hypochlorite
Hydrogen Sulfide (H2S) Lewis Structure. Lewis structure of Hydrogen sulfide (H 2 S) contains two S-H single bonds around sulfur atom. Also, there are two lone pairs around sulfur atom. Concept of number of total valence electrons of sulfur and hydrogen atoms are used to draw lewis structure of H 2 S. Each step of drawing lewis structure of H 2 S is explained in detail in this tutorial.

0 Response to "36 lewis dot diagram for h2s"
Post a Comment