7 lewis dot diagram for selenium
Selenium element is located in Group 6A of the periodic table. This in dicates that it has 6 electron in its valence or outermost shell. The Lewis dot structure of Selenium shoulde be written with Se surrounded by six dots. These six dots don't have to be an all four sides but if you do then that is the conventional way of doing it. Because selenium is in the 16th column on the periodic chart, it has 6 valence electrons. Thus, the Lewis structure (or electron dot structure) for selenium has 6 dots around it. It will look ...
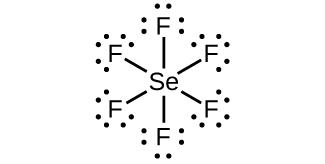
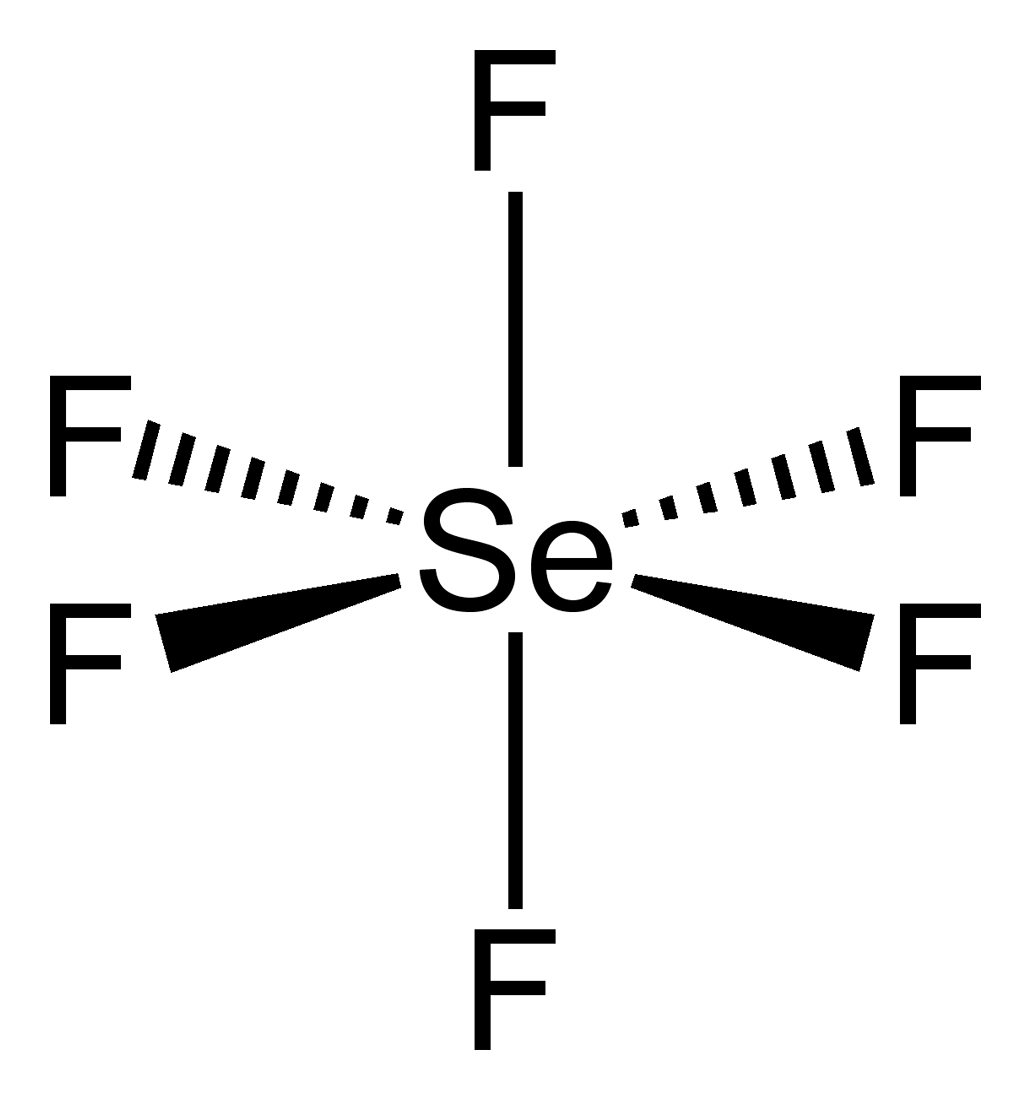
Selenium tetrafluoride (SeF4) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Selenium tetrafluoride is an inorganic compound that appears as a colorless liquid having the chemical formula SeF4. It can react with water and forms hydrofluoric acid and selenous acid. Selenium in the SeF4 molecule has a +4 oxidation state.

Lewis dot diagram for selenium
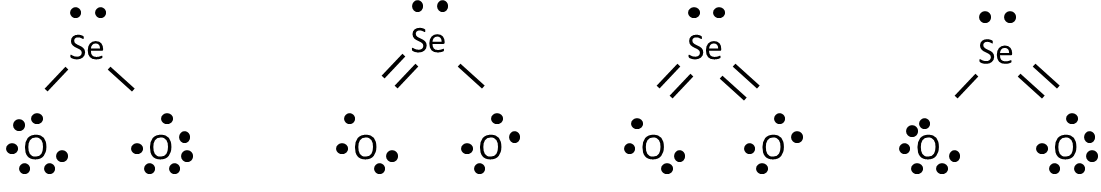
Lewis dot structure is the structure which represent the valence electrons of the element around the chemical symbol of the element. Selenium is the 34th element of the periodic table. Atomic number of bromine is 34 that means it contains 34 electrons, Thus the electronic configuration is represented as: Thus the valence electrons are 6. Lewis Diagram For Seo3 A Lewis dot structure for SeO3 is drawn with an Se in the center, with two lines connecting it to two Os and one double line connecting it to an O. The Os. Draw all possible resonance structures for the molecule selenium trioxide (SeO3) . Example \(\PageIndex{1}\): What is the Lewis electron dot diagram for each element? aluminum; selenium; Solution. The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: \[\dot{Al:} \nonumber\nonumber \]
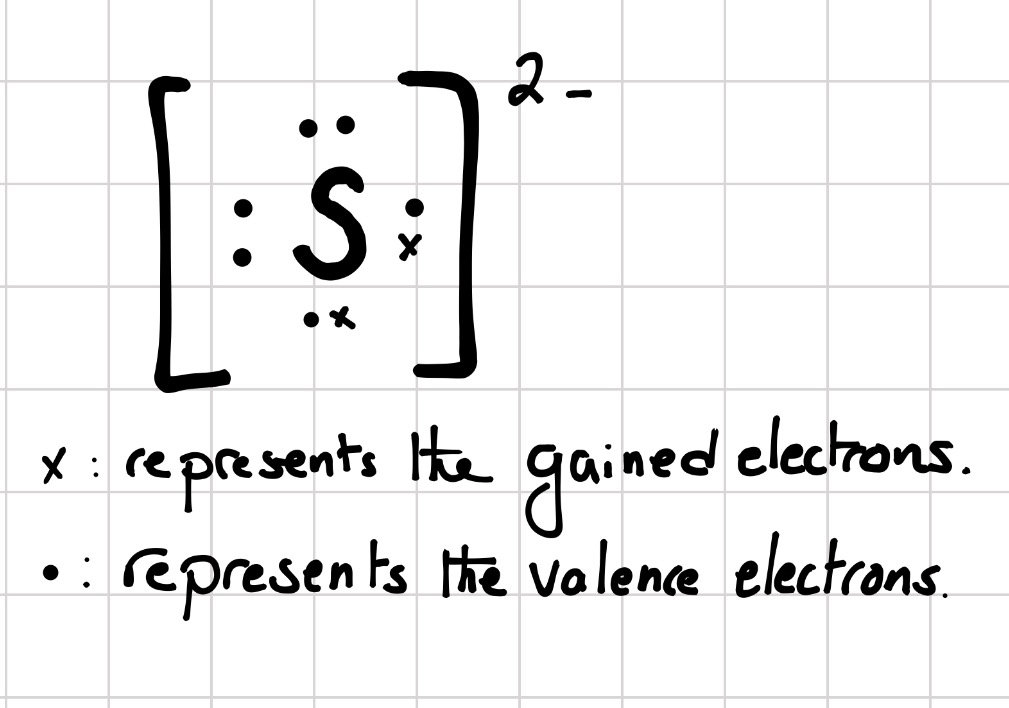
Lewis dot diagram for selenium. Because selenium is in the 16th column on the periodic chart, it has 6 valence electrons. Thus, the Lewis structure (or electron dot structure) for selenium has 6 dots around it. Example 1. What is the Lewis electron dot diagram for each element? a) aluminum b) selenium . Solution. a) The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: b) The valence electron configuration for selenium is 4s 2 4p 4. What is the Lewis electron dot diagram for each element? aluminum selenium Solution The valence electron configuration for aluminum is 3 s2 3 p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons: The valence electron configuration for selenium is 4 s2 4 p4. A step-by-step explanation of how to draw the Se (Selenium) Se2- Selenide ion) Lewis Dot Structure.For the Se and Se 2- structure use the periodic table to...
Example \(\PageIndex{1}\): What is the Lewis electron dot diagram for each element? aluminum; selenium; Solution. The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: \[\dot{Al:} \nonumber\nonumber \] Lewis Diagram For Seo3 A Lewis dot structure for SeO3 is drawn with an Se in the center, with two lines connecting it to two Os and one double line connecting it to an O. The Os. Draw all possible resonance structures for the molecule selenium trioxide (SeO3) . Lewis dot structure is the structure which represent the valence electrons of the element around the chemical symbol of the element. Selenium is the 34th element of the periodic table. Atomic number of bromine is 34 that means it contains 34 electrons, Thus the electronic configuration is represented as: Thus the valence electrons are 6.











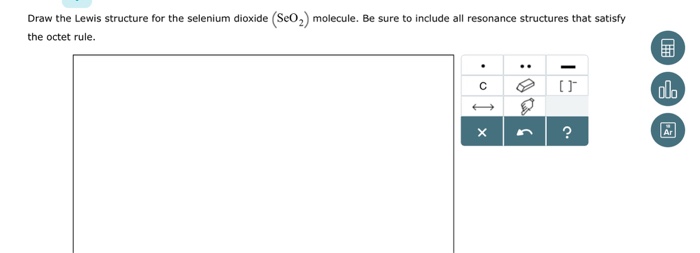
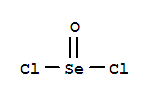

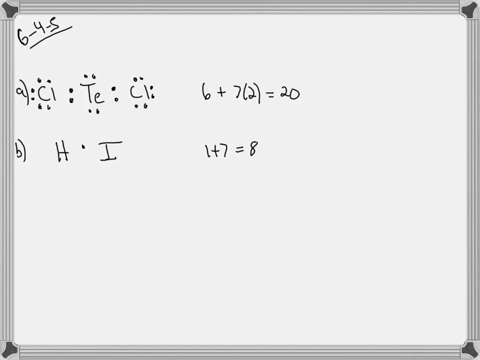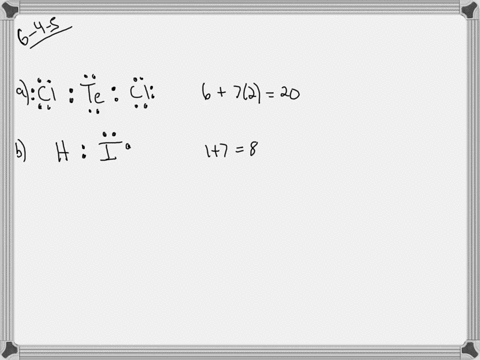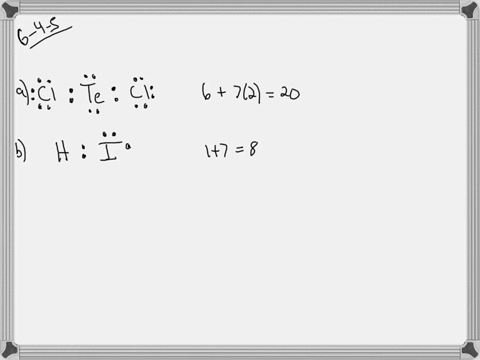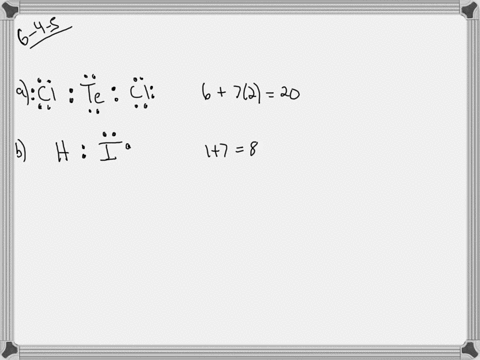



0 Response to "7 lewis dot diagram for selenium"
Post a Comment