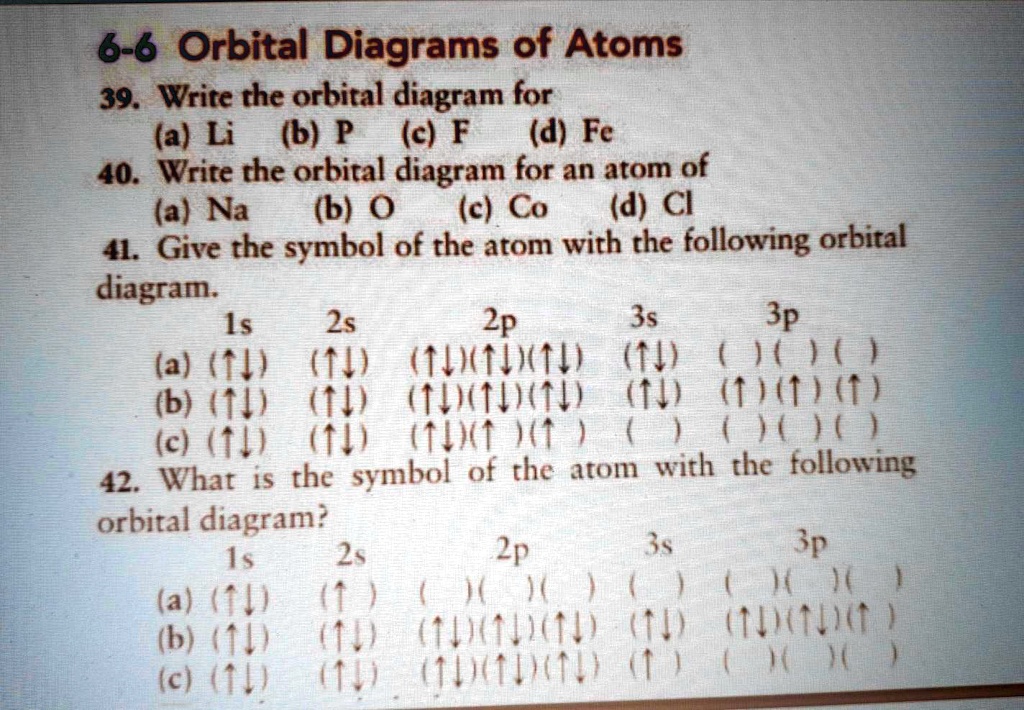
40 orbital diagram of fe
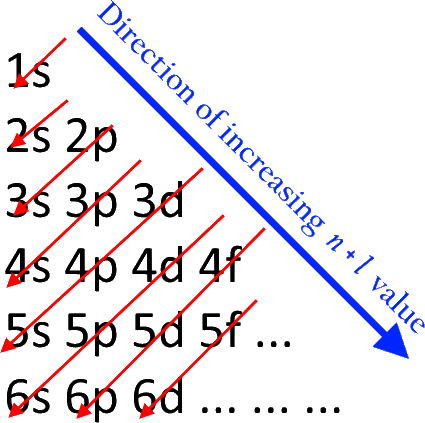
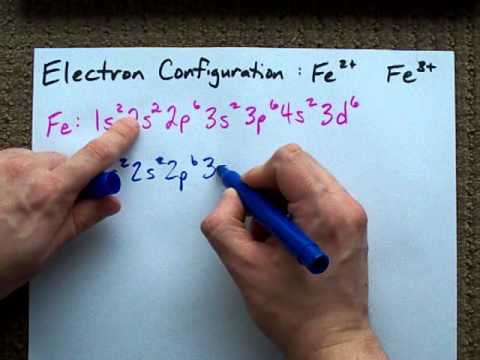
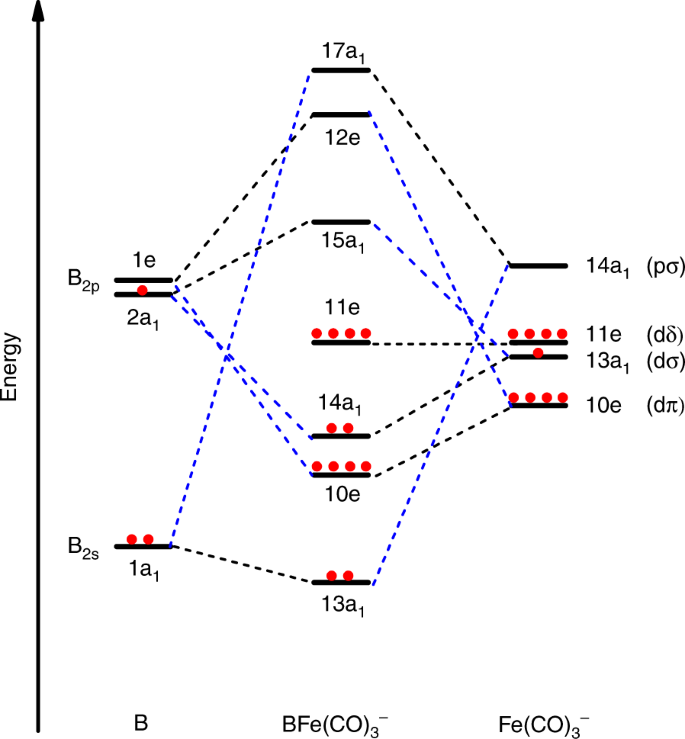
What is electron configuration of Fe? [Ar] 3d6 4s2. How many d electrons are in FE? From the periodic table, iron has atomic number 26 , meaning that there are 26 electrons in each ground state iron atom. 2⋅5=10 electron in each d orbital, and so on so forth. For clarity, hydrogen atoms are not shown. Electronic d-orbital diagram of the Fe V center is shown, as derived from DFT. Selected bond lengths: Fe–O, 1.60 Å; Fe–N, 1.86 Å (two nitrogens along x direction) and 1.91 Å (two nitrogens along y direction); the Fe is 0.5 Å above the plane defined by the four amide nitrogens.
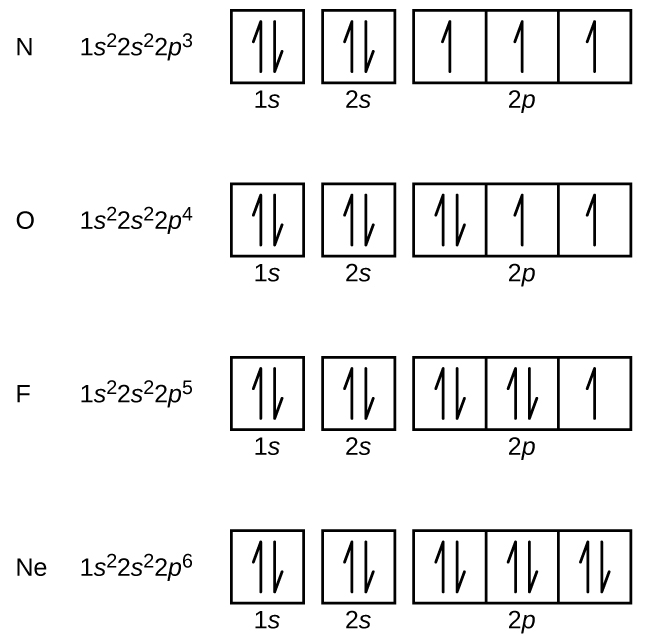
4. Spin Quantum Number (ms): m s = +½ or -½. Specifies the orientation of the spin axis of an electron. An electron can spin in only one of two directions (sometimes called up and down). The Pauli exclusion principle (Wolfgang Pauli, Nobel Prize 1945) states thatno two electrons in the same atom can have identical values for all four of their quantum numbers.
Orbital diagram of fe
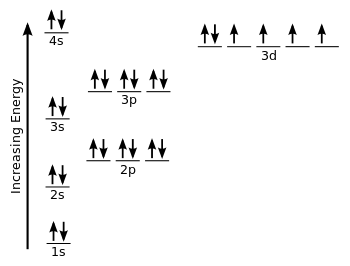
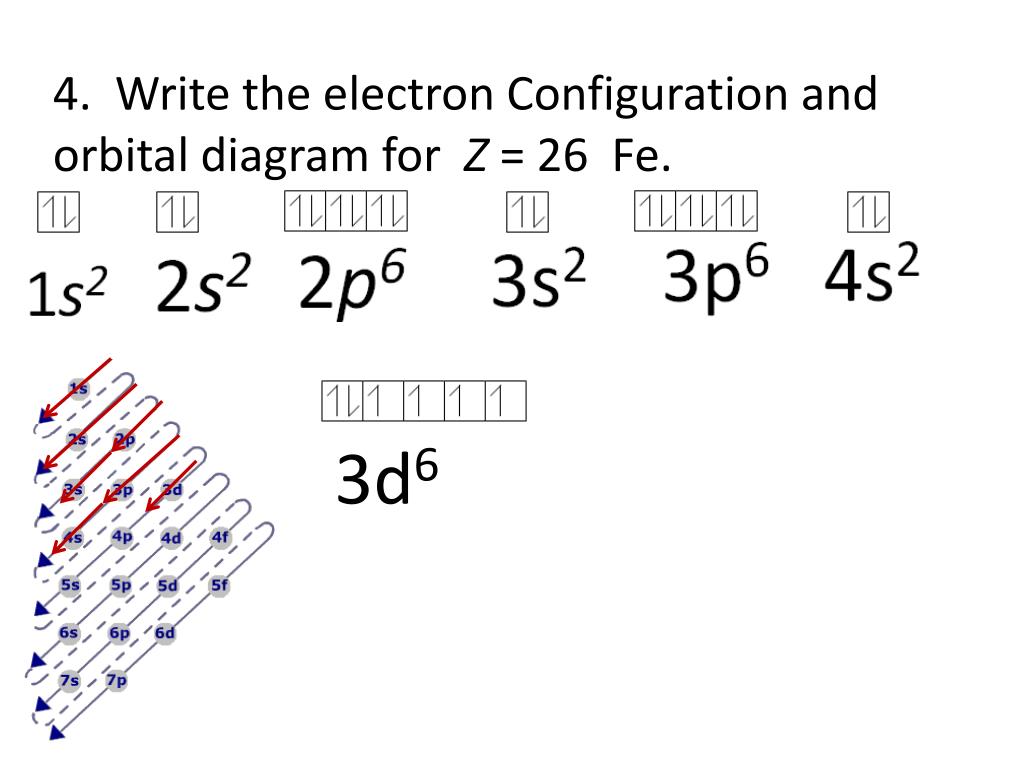
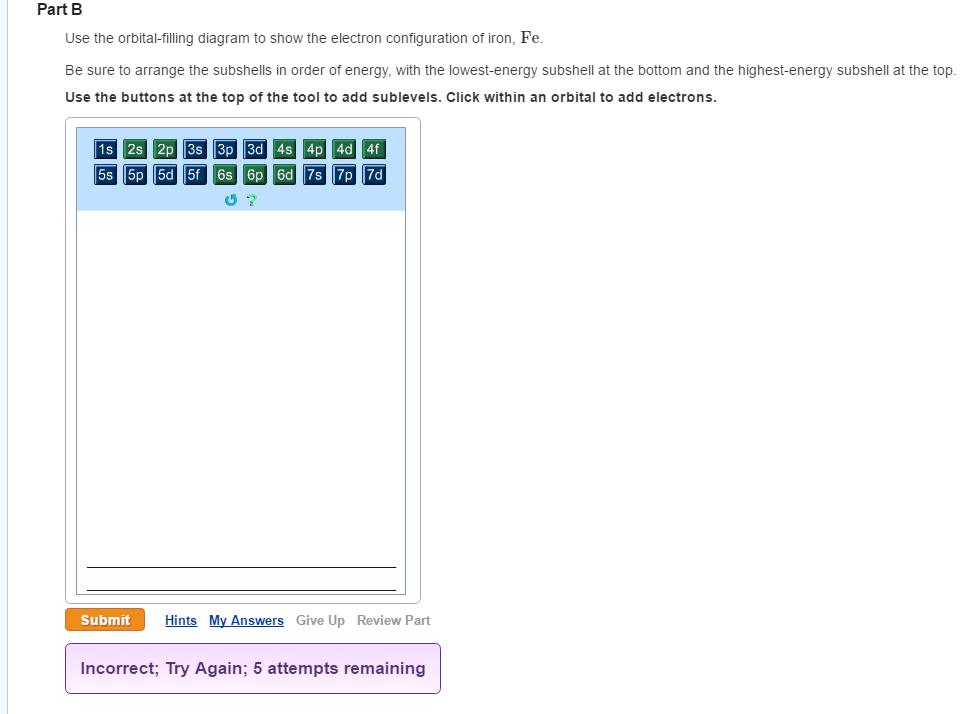
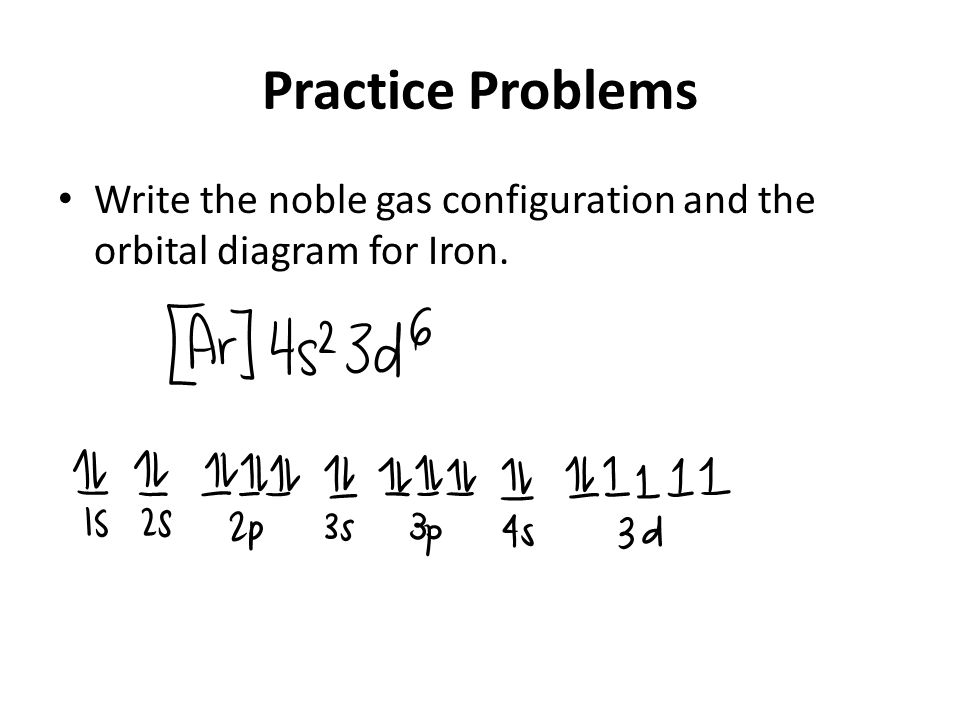
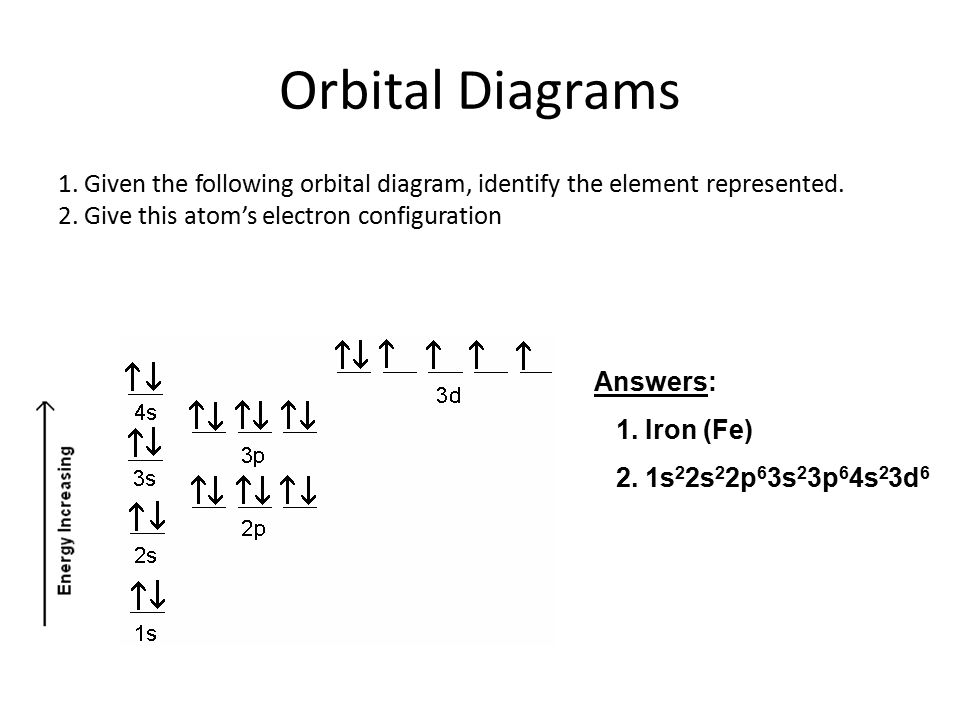
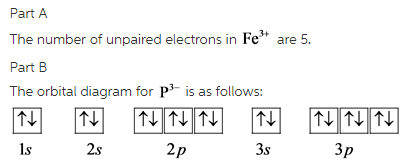
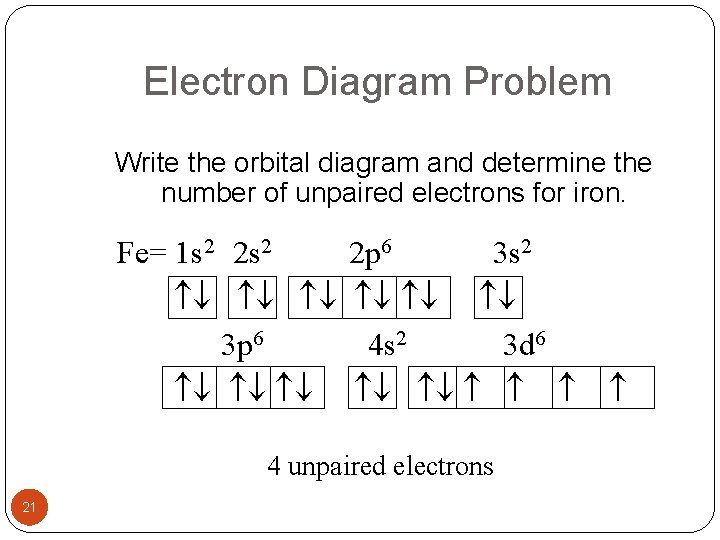
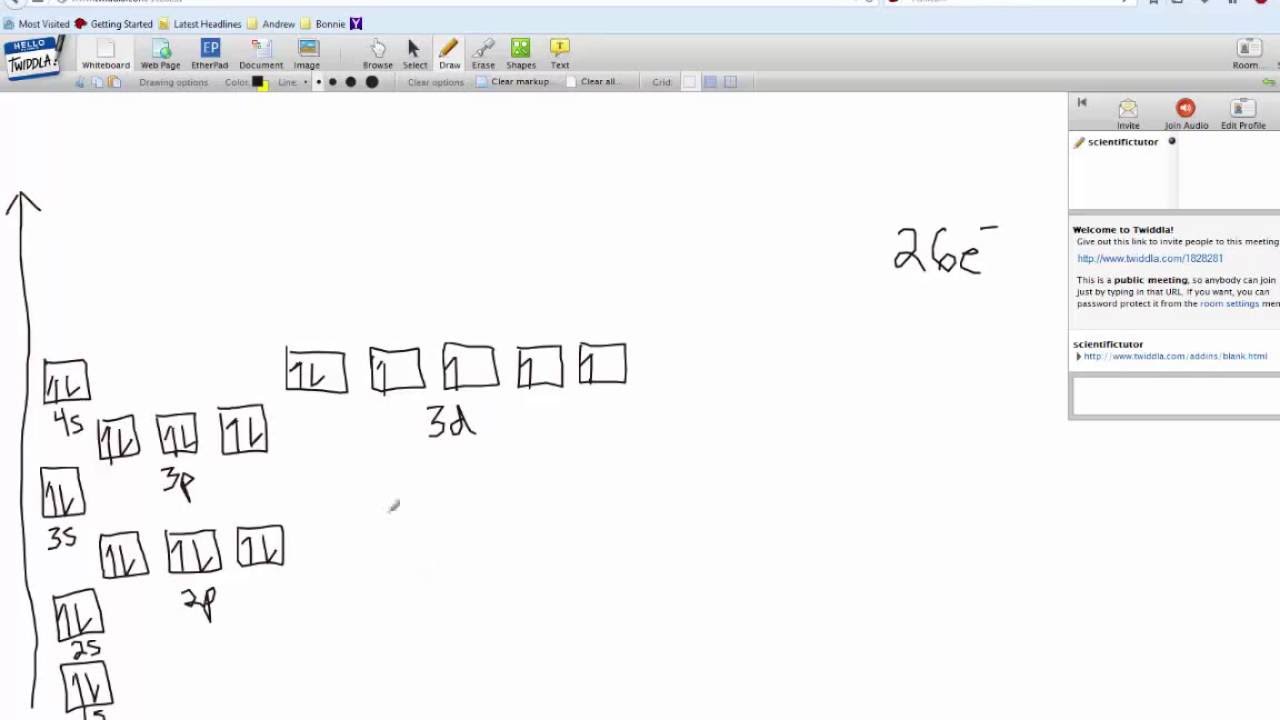
Atomic Orbital Diagram for Iron(Fe) Iron ion(Fe 2+,Fe 3+) electron configuration. Ground state electron configuration of iron(Fe) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2.The electron configuration shows that the last shell of iron has two electrons and the d-orbital has a total of six electrons. Fe, or iron, has the atomic number of 26. Its full orbital diagram is 1s2 2s2 2p6 3s2 3p6 4s2 3d6. STEEL and Fe–C PHASE DIAGRAM 22. An Fe-C alloy with 0.395 weight % carbon is austenitized at 1000 C and very slowly cooled to 728 C. What is the amount (weight percent) of austenite present in the microstructure at 728 C? (A) 25 (B) 40 (C) 50 (D) 100
Orbital diagram of fe. What is the orbital diagram for Fe? - Answers Fe, or iron, has the atomic number of 26. diagram is 1s2 2s2 2p6 3s2 3p6 4s2 3d6. Home Study Guides Science Math and Arithmetic History Literature and... Download scientific diagram | (a) Calculated total and orbitally resolved exchange parameters of Fe-Fe atoms as a function of distance. The positive and negative values indicate ferromagnetic and ... Electronic Configuration of Iron. Iron is a chemical element with an atomic number 26. A symbol Fe represents it. It is the most common element that is found on the earth. Unlike that of other elements, iron exists at oxidation states of -2 to +6. Elementary iron occurs in a low-oxygen environment even though it is reactive to water and oxygen. After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of ...
STEEL and Fe–C PHASE DIAGRAM 22. An Fe-C alloy with 0.395 weight % carbon is austenitized at 1000 C and very slowly cooled to 728 C. What is the amount (weight percent) of austenite present in the microstructure at 728 C? (A) 25 (B) 40 (C) 50 (D) 100 Fe, or iron, has the atomic number of 26. Its full orbital diagram is 1s2 2s2 2p6 3s2 3p6 4s2 3d6. Atomic Orbital Diagram for Iron(Fe) Iron ion(Fe 2+,Fe 3+) electron configuration. Ground state electron configuration of iron(Fe) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2.The electron configuration shows that the last shell of iron has two electrons and the d-orbital has a total of six electrons.

![Walsh diagram for [Fe(Por)(NO)]. The top part of the orbital ...](https://www.researchgate.net/profile/Abhik-Ghosh-3/publication/44661768/figure/fig7/AS:319435861708814@1453170852610/Walsh-diagram-for-FePorNO-The-top-part-of-the-orbital-energy-scale-is-expanded-35.png)
/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)

















0 Response to "40 orbital diagram of fe"
Post a Comment