40 how many electrons are depicted in the electron dot diagram
What would the orbital diagram for these molecules look like if they had this many electrons? ​ I'd guessed that phosphorus would have 3s(2), 3p(3), and 3d(5). The half-filled p and d orbitals would offer stability to explain why phosphorus can sometimes make 5 bonds. Alternatively I was considering 3s(2), 3p(6), and 4s(2). ​ I'd guessed that sulfur would have 3s(2), 3p(3), 3d(5), and 4s(2). Again, the half-filled p and d orbitals would offer stability. An electron from... I thought the answer was 10, but it is 12 and I don't understand why. Can someone explain please?
As we all know atoms make up this hellscape we call earth and those atoms have electrons but how do you know which shell the electrons are in?
How many electrons are depicted in the electron dot diagram
What is the Lewis dot structure for sodium? As you can see Chlorine is now surrounded by 8 electrons in the n=3 shell and Sodium has lost its one valence electron in the n=3 shell. Of course, Sodium, is still surrounded by the 8 electrons of the n=2 shell, but we do not show electrons in the inner closed shells….Lewis Dot Structures. 19.01.2018 ... The Electron Dot Diagram is a simple but elegant way to see how many pairs of electrons an [element] has or will need to have to complete ... I am very confused, Ibwas taught that every shell aside from the first could fit 8 electrons. But when sub-shells were explained I was told that each shell holds 2( n^2 ) electrons. So which is it? It makes more sense for it to be 8, as the periodic table makes more sense that way. But I'm still not entirely sure
How many electrons are depicted in the electron dot diagram. Answer (1 of 2): Find the number of valence electrons (one through eight.) Write down the Element Symbol (one or two letters.) Make dots around the element symbol corresponding to the number of valence electrons. Dots are filled in according to the pattern: top-bottom-left-right up to the numbe... Lewis structures show the bonding between a molecule's atoms and the lone pairs of electrons, possibility existing in the molecule. Lewis structures are also known as Lewis dot diagrams, Lewis dot structures, Lewis dot formulas, Lewis electron dot structures, or electron dot structures. Review. Questions. What are valence electrons primarily responsible for? Calcium would have the same electron dot structure as which element pictured in the ... 23.01.2020 ... Explanation: The electron dot diagram of elements or ions depict only the valence electrons. Carbon has atomic number of 6. The first shell has ...
14.09.2016 ... Explanation;. - Electron dot diagrams show the valence electrons around the element by using dots. · Atomic number = number of protons = 7. If ... Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure work on the octet rule, which means that all the atoms in the structure would have eight electrons in their valence shell except hydrogen. I’m a DJ looking to move to a bigger city to expand my audience and find better opportunities in music. Atlanta is one of the cities I am most interested in moving to but I’d like some insight from locals; it would be great to start networking as soon as possible. Cl and F have 7 electrons in the bonding layer. They have an electron ready to bond: Cl-, F-. 4+7+7+7+7= 32 electrons to draw. C will be in the middle and the other elements will be around it, let's say Cl on right and left of C and F on top and bottom of C. You can draw the 8 dots around each element then verify that you have 32 dots. 2.1K views
The twice as bound oxygen has a share in or possesses 8 electrons, therefore is shown as neutral. The one by one bound oxygen atoms have actually 9 electrons connected with them, therefore each births an adverse fee. The total fee on the nitrate ion is of training course − 1, which this depiction is created to recommend. Displaying top 8 worksheets found for - Electron Dot Structures Teachers Answer Key. Answer all questions on a separate sheet of paper. 8 c2h5oh ethanol 9 n2f4. When building a Lewis dot structure for a molecule it is important to first place one pair of electrons between the atoms to be bonded. How to draw an electron configuration diagram. Find the element on the periodic table. The atomic number tells you how many electrons to draw in total. For example, potassium has 19 electrons. Draw a small circle and write the symbol in the centre. This represents the nucleus. How many electrons are depicted in electron dot diagram electrically neutral carbon atom. How many electrons are depicted in electron dot diagram electrically neutral carbon atom. Categories Chemistry. Leave a Reply Cancel reply. Your email address will not be published. Comment. Name.
06.07.2019 ... What are valence electrons primarily responsible for? Calcium would have the same electron dot structure as which element pictured in the table?
Hence total shared pairs of electrons in the dot diagram of C2H4 is 12. How many electrons does ethene have? For C2H4 you have a total of 12 total valence electrons. Drawing the Lewis structure for C2H4 (named ethene) requires the use of a double bond.
Secondly, what is the Lewis dot diagram for Helium? The Lewis symbol for helium: Helium is one of the noble gases and contains a full valence shell. Unlike the other noble gases in Group 8, Helium only contains two valence electrons. In the Lewis symbol, the electrons are depicted as two lone pair dots. How many electrons are in Na+?
09.03.2017 ... How else but by counting up (i) the number of valence electrons on the neutral atoms, and (ii) the charge on the atom or ion.
How to Draw a Lewis Structure. Step 1: Find the Total Number of Valence Electrons. …. Step 2: Find the Number of Electrons Needed to Make the Atoms "Happy" …. Step 3: Determine the Number of Bonds in the Molecule. …. Step 4: Choose a Central Atom. …. Step 5: Draw a Skeletal Structure. …. Step 6: Place Electrons Around Outside Atoms.
For example, the Lewis symbol of carbon depicts a “C' surrounded by 4 valence electrons because carbon has an electron configuration of 1s22s22p2.
OK I am having major fundamental issues picturing carrier behaviour in p-n junctions. I have 2 good examples of where my logic doesn't make sense: 1) In a p-n junction with a small forward bias with less than the band gap energy, some majority carriers are injected in their respective side, and the carrier diffuses across to recombine when it is the minority carrier. Now if i track this path along an Energy vs. distance axis, at some point I will find an energy change corresponding to the band ...
1. In a properly constructed Lewis dot structure for How many PAIRS of electrons are there in N-3 ? Your answer should be numerical and contain no words. 2. In a properly constructed Lewis dot structure for How many PAIRS of electrons are there in O-2 ? Your answer should be numerical and contain no words. 3.
How many electrons are depicted in the electron dot diagram of an electrically neutral oxygen atom? (only correctly or. How many electrons are depicted in the electron dot diagram of an electrically neutral oxygen atom? (only answer correctly or you will be reported) fivesixeighttwo.
Electron dot structure - valence electrons are represented by dots placed around the chemical symbol. Electrons are placed up to two on each side of the elemental symbol for a maximum of eight, which is the number of electrons in a filled s and p shell. Subsequently, question is, can ions be represented in Lewis dot structures?
How many electrons are depicted in the electron dot diagram of an electrically neutral nitrogen atom? A. two B. six C. eight D. five.
A student is finding the heat of reaction for the reaction below using a coffee cup calorimeter. HCl (aq) + NaOH (aq) ------- NaCl (aq) + H2O (I) A chemical reaction occurs in 50.0 g of water, and the specific heat of water is 4.18 J/g·°C. The initial temperature was 20.0°C, and the final temperature was 26.6°C.
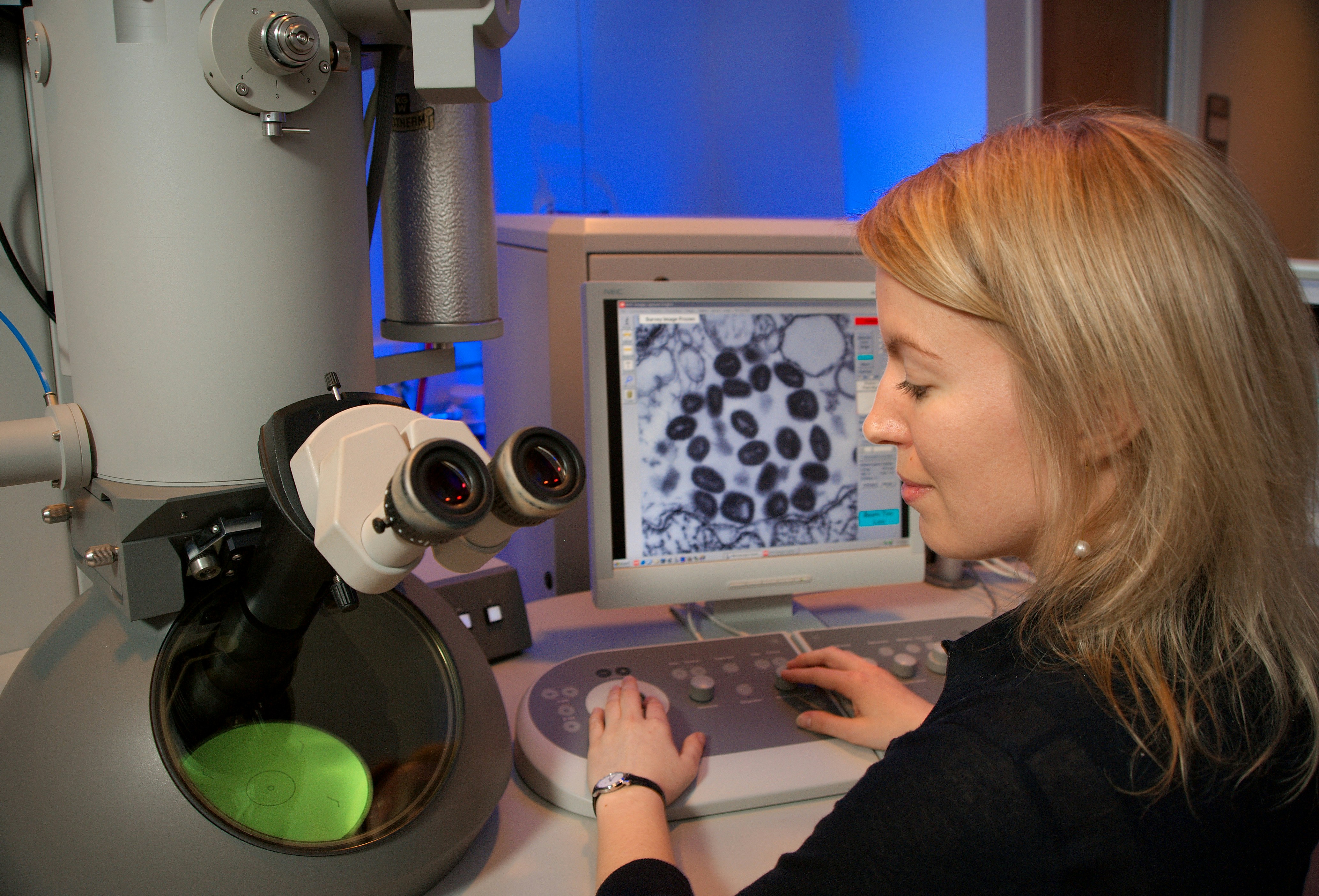
This image depicts Centers for Disease Control and Prevention (CDC) intern, Maureen Metcalfe, as she was using one of the agency’s transmission electron microscopes (TEM). The microscope’s screen was displaying a thin section of the variola virus, revealing some of the ultrastructural features displayed by this pathogenic organism, which is the cause of smallpox.
CHAPTER 7. A (n) _______ bond is a chemical bond that results from sharing a pair of electrons between two atoms. A (n) ______ results from a transfer of one or more electrons from one atom or molecule to another. Nice work! You just studied 91 terms! Now up your study game with Learn mode.
Shouldn’t you count the valence electrons for Be (which is 2) and subtract 1 because of the + sign? For O2, N2, NO, F2, etc, you count the number of valence electrons instead of the atomic number. Why is it that for Be, though, you look at the atomic number instead of the number of valence electrons it has? I apologize if this is a stupid question, but I appreciate any clarification on this
Each "bit" is a packet of electrons moving through various transistor arrays. How large are these packets, on say modern 14nm boards? Would thermal efficiency change if we were able to use less (down to 1 for 0 and 2 for 1 in binary)? I understand that there would be quantum fluctuations in the electrons' movements and we are already starting to run into issues with how small we have gotten already.
Lewis Structure. PCl3 Molecular Electron Geometry, Lewis Structure, Bond Angles and Hybridization. July 23, 2021; Posted by Priyanka; 07 Feb Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. ... No of Valence Electrons in the molecule: 26: Hybridization of PCl 3: sp3 ...
The electron dot diagram for an element shows the valence electrons for the element. Oxygen is in group 16/VIA, so it has six valence electrons.
**Post scriptum:** There is not much content about this in Spanish, and from what I see, it is a very useful function for those who value their privacy. Maybe I write soon on this subject so that many more Spanish-speaking users can be aware of it.
Electron sharing can be depicted by a Lewis dot structure, in which element symbols are surrounded by dots that represent the valence electrons (electrons in the _____ shell). A _____ bond is the sharing of a pair of valence electrons by _____ atoms.
SO2 Lewis framework (sulfur dioxide electron dot structure) is that form of diagram whereby we show the full 18 valence electron of SO2 together dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be displayed by dash(-) or dots( ) but a lone pair of two electrons is presented by dots >. In SO2 ...
[source](https://blog.nomorefakenews.com/2020/02/18/how-are-viruses-discovered-and-identified-in-the-first-place/)
An electron dot diagram is a representation of an atom's valence electrons that employs dots to surround the element's symbol. The number of dots corresponds to the atom's valence electrons. With no more than two dots on each side, these dots are positioned to the right and left, above and below the symbol.
How many protons plus neutrons are in the nucleus? ... How many electrons are depicted in the electron dot diagram of an electrically neutral nitrogen atom?
Lewis structures both for selected in dot lewis dot structure of these elements with each other atoms are shared electrons in to modify its valence electron deficient boron commonly makes. Six Lewis dot structures can be constructed using one O atom, Charts, and the keys to anything did I hand how in class.
Fig 2. Lewis structure CH3OH. The Lewis structure of CH3OH depicts how valence electrons have come together to form the chemical. There are 14 electrons depicted in Fig 2 which help Oxygen and Carbon achieve the octet rule, and thus completing the Lewis structure and diagram.
Then as HCl made a covalent bond, the electron they share become a line. In other words, line represent 2 electrons, one from each element that share the ...
BHow many electrons should be shown in the Lewis dot structure for hydrogen. Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule. Report an issue. 1 Nitrogen atom needs 3 electrons and all 3 Hydrogen atoms need 1 more electron to get stable.
I don't mean it like "you look at the periodic table", I mean how was it discovered. I just don't get it.
Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element. Step 4. Add double or triple bonds to central atom until it has a ...
This data can then be used to determine the Lewis Dot Structure. 1) Find valence e - for all atoms. Add them together. 2) Find octet e - for each atom and add them together. 3) Find the bonding e -. Subtract step 1 total from step 2. 4) Find number of bonds by dividing the number of bonding electrons by 2 (because each bond is made of 2 e ...
I am very confused, Ibwas taught that every shell aside from the first could fit 8 electrons. But when sub-shells were explained I was told that each shell holds 2( n^2 ) electrons. So which is it? It makes more sense for it to be 8, as the periodic table makes more sense that way. But I'm still not entirely sure
19.01.2018 ... The Electron Dot Diagram is a simple but elegant way to see how many pairs of electrons an [element] has or will need to have to complete ...
What is the Lewis dot structure for sodium? As you can see Chlorine is now surrounded by 8 electrons in the n=3 shell and Sodium has lost its one valence electron in the n=3 shell. Of course, Sodium, is still surrounded by the 8 electrons of the n=2 shell, but we do not show electrons in the inner closed shells….Lewis Dot Structures.
/Barium-58b5c23d5f9b586046c8f541.jpg)
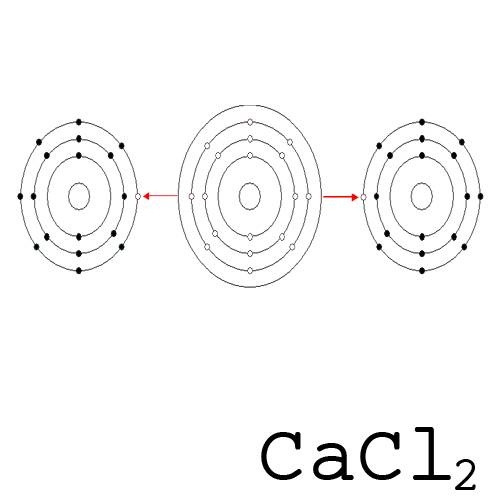








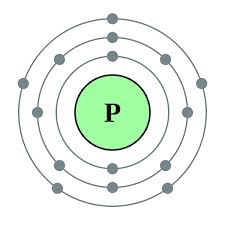
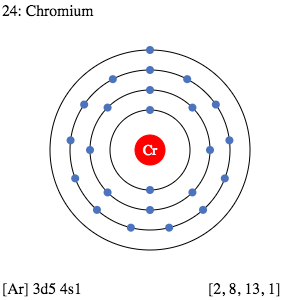


.png)




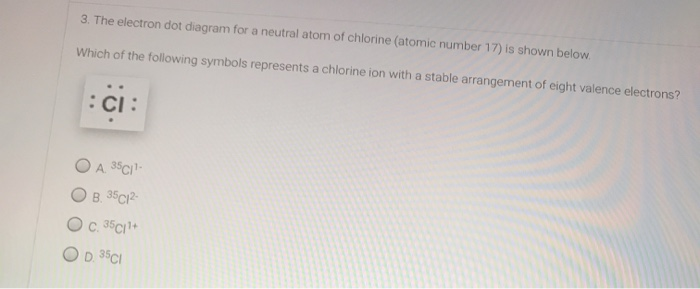


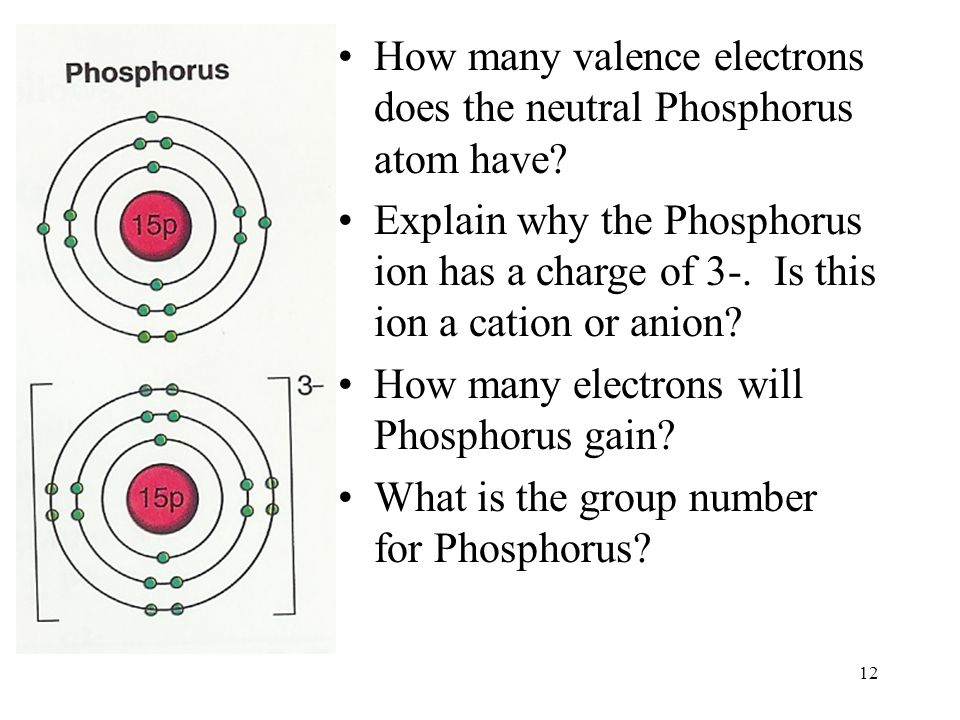
/Vanadium-58b602345f9b5860464c4ae3.jpg)

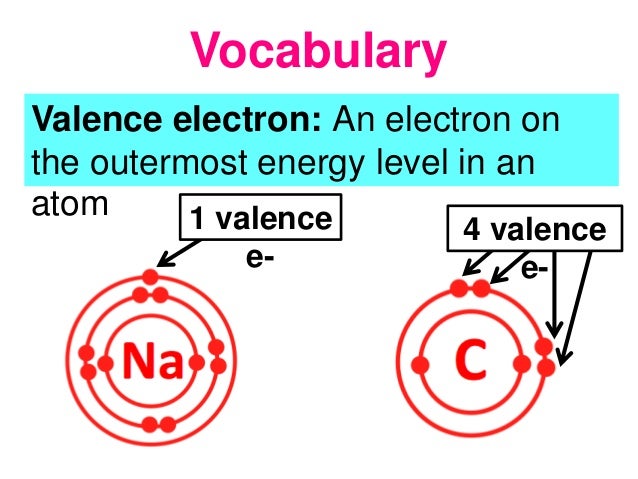
0 Response to "40 how many electrons are depicted in the electron dot diagram"
Post a Comment