39 partial energy level diagram for hydrogen
For energy levels, we use ideal hydrogen atom values. For atomic line transitions , we use values tabulated by the National Institute of Science & Technnology (NIST) (formerly the National Bureau of Standards (NBS) ) ( Wiese et al. 1966, p. 2 ; Wiese et al. 1966, p. 2, online ). Fig. 6-1. Energy level diagram of some of the excited states of the 12C nucleus. The angular momentum (J), parity (P), and isospin (T) quantum numbers of the states are indicated on the left using the notation J P. P and n respectively at the top of the diagram indicate the separation energies for a proton and a neutron.
Related Threads on Partial Energy Level Diagram for Hydrogen Energy level diagram of a chemical reaction. Last Post; Jul 30, 2020; Replies 4 Views 728. Energy in Liquid Hydrogen. Last Post; Apr 30, 2010; Replies 1 Views 2K. Energy state of hydrogen molecule. Last Post; Aug 1, 2012; Replies 1 Views 3K. P. Hydrogen bonds and binding energy ...

Partial energy level diagram for hydrogen
With neon the second level is filled with electrons. Completed levels are a characteristic of all noble gases. If we look at the energy level diagram for neon the completed second level means the next electron must go into the third level. In the hydrogen atom the three sublevels, 3s, 3p and 3d were all degenerate in energy. Click here👆to get an answer to your question ️ The following question relates to the partial energy level diagram for the hydrogen electron: .54eV n5 .85eV n4 1.51eV n3 3.4eV n2 13.6eV n1 The question relates to electron located at E3 . What is the emission energy when the electron falls to E1 from E3 ? electron of a hydrogen atom can occupy only certain energy levels. (Think of energy levels as unequally spaced steps of a ladder, with a huge 1st step and sequentially smaller steps thereafter. The higher up an electron is on the ladder, the more energy it has.) Astronomers use the letter 'n' and a number to designate each energy level.
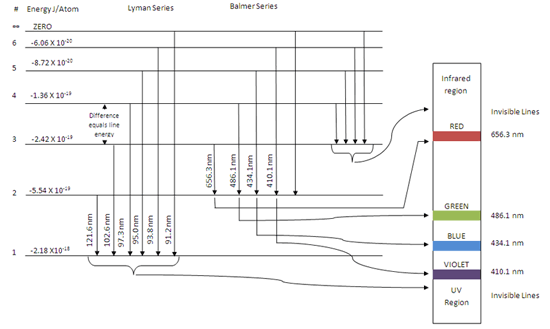
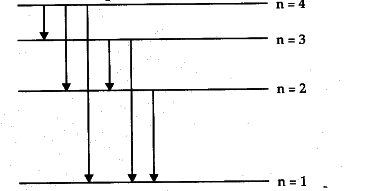
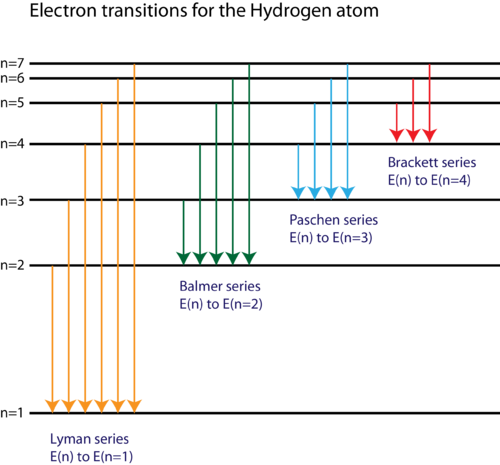
Partial energy level diagram for hydrogen. For hydrogen, the ionization energy = 13.6eV When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon. The Lyman (ultraviolet) series of spectral lines corresponds to electron transitions from higher energy levels to level n = 1. Energy level diagrams for IONS Atoms with 1, 2, or 3 valence electrons lose them to form 1+, 2+ or 3+ ions respectively. naming metallic ions - the full name of the atom is followed by the word ion. Mg2+ is the magnesium ion Group 1 (1+) (lose 1e) Group 2 (2+) (lose 2e) Group 13 (3+) (lose 3e) Energy level diagrams for IONS A Partial Molecular Orbital Energy-Level Diagram for the HF Molecule A Partial Molecular Orbital Energy-Level Diagram for the HF Molecule Because the fluorine 2p is lower in energy than the hydrogen 1s, the electrons spend more time near the fluorine nucleus. Figure 9.44 ... MIT 8.04 Quantum Physics I, Spring 2016View the complete course: http://ocw.mit.edu/8-04S16Instructor: Barton ZwiebachLicense: Creative Commons BY-NC-SAMore ...
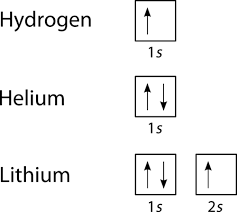
The simplest energy level diagram at intermediate pressure used in SWD modeling includes the Ar atom in ground state and the 4s block of levels [42]. We are using the energy level diagram of the ... Give the ground-state electron configuration, symbol, and group number from each partial (valence-level) orbital diagram. A zinc atom in its ground state has how many unpaired electrons? ... (energy levels) of a hydrogen atom vary as s < p < d, etc. e. the energies of subshell in the shells (energy levels) of a hydrogen atom vary as s < p < d ... Notice how each energy level closer and closer to the nucleus is more and more negative. This signifies that the electron is trapped in an "energy well." To ionize a ground-state electron [to take it from -122.4 eV to 0 eV in our example], you would have to irradiate the gas with photons having energies of 122.4 eV or greater. The emission spectrum of hydrogen Energy levels of the hydrogen atom: De-excitation of electron results in emission of photon-13.6 eV 0.0 eV E PHYS 1493/1494/2699: Exp. 7 - Spectrum of the Hydrogen Atom
Click here to get an answer to your question ✍️ The following question relates to the partial energy level diagram for the hydrogen electron: .54eV n5 ...1 answer · Top answer: Energy is released when an electron jumps from a higher energy level to a lower energy level, and released energy is the difference in the total energy ... Lithium energy-level diagram Energy level diagrams for the easily excited atomic lines of lithium, sodium, potassium and rubidium. Wavelengths are given in nanometres for the spectral lines produced by transitions between the different levels. The ionization potential is indicated by the dashed line above the respective diagrams. The Selection Rule for L - The energy-level diagram for lithium ... level diagram, or potential energy profile, as shown in Figure 13.1. The vertical axis gives the potential energy for the reaction, while the horizontal axis is a relative (i.e., time) scale that shows the progress of the reaction. The diagram indicates that there is a "hill" or energy barrier that needs to be overcome before any products ... Although hydrogen has only one electron ,there are still many different energy-level transitions that electron can make; thus, hydrogen shows many different absorption lines. Physicists classify these lines based on the (low) energy level the electron begins on (before a photon is absorbed). Absorption lines caused by electrons starting in the ...
Draw a partial energy level diagram for hydrogen. All wavelengths are ending at the n = 2 state and the energy of the n = 2 state is -.545 aJ. Wavelengths(nm): 411.26, 434.77, 487.10, 658.42
Sodium energy-level diagram Energy level diagram of the sodium atom.The energy levels are denoted by the values for the principal quantum number, the orbital quantum number/, and the spin quantum number s. Levels with 1 = 0 are not split for / = 1 two separate levels are drawn (s = 1/2) for/> 1 the splitting is too small to be shown in the figure.
5.13 The energy level diagram for SH- is shown below. A bond order of 1 is predicted. The S orbital energies are -22.7 eV (3s) and -11.6 eV (3p); the 1s of H has an energy of -13.6 eV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the
Partial Energy Level Diagram for Hydrogen - Partial Energy Level Diagram for Hydrogen 1 = (6.626 x 10-37 KJ (3.00 x 1017 nm\/s (6.022 x 1023 atoms\/mole = | Course Hero Partial Energy Level Diagram for Hydrogen - Partial Energy... School Cornell University Course Title CHEM 2070 Type Lab Report Uploaded By ProfessorResolveBadger1305 Pages 1
This chemistry video tutorial focuses on the bohr model of the hydrogen atom. It explains how to calculate the amount of electron transition energy that is...
Line Color 2 (nm) v (Hz) nl AE (J) E (1) 2 4.9 x 10-19 -0.6 x 10-19 410 7.3 x 1014 6. Partial Energy Level Diagram for Hydrogen: Energy (J/atom) -1 x 10-19 -2x 10-19 -3 x 10-19 -4 x 10-19 -5 x 10-19 -6 x 10-19 Previous question Next question
Energy level diagrams and the hydrogen atom It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states.
electron of a hydrogen atom can occupy only certain energy levels. (Think of energy levels as unequally spaced steps of a ladder, with a huge 1st step and sequentially smaller steps thereafter. The higher up an electron is on the ladder, the more energy it has.) Astronomers use the letter 'n' and a number to designate each energy level.
Click here👆to get an answer to your question ️ The following question relates to the partial energy level diagram for the hydrogen electron: .54eV n5 .85eV n4 1.51eV n3 3.4eV n2 13.6eV n1 The question relates to electron located at E3 . What is the emission energy when the electron falls to E1 from E3 ?
With neon the second level is filled with electrons. Completed levels are a characteristic of all noble gases. If we look at the energy level diagram for neon the completed second level means the next electron must go into the third level. In the hydrogen atom the three sublevels, 3s, 3p and 3d were all degenerate in energy.











.png?revision=1)












0 Response to "39 partial energy level diagram for hydrogen"
Post a Comment