39 molecular orbital diagram for h2o
Mercury is the smallest planet in the Solar System and the closest to the Sun.Its orbit around the Sun takes 87.97 Earth days, the shortest of all the Sun's planets. It is named after the Roman god Mercurius (), god of commerce, messenger of the gods, and mediator between gods and mortals, corresponding to the Greek god Hermes (Ἑρμῆς). Like Venus, Mercury orbits the Sun within Earth's ... Hi I am a PhD candidate and I need help making a frontier molecular orbital visualization diagram for the molecule pentacene. I only need 4 molecular orbital visualizations and they are HOMO, LUMO, HOMO-1, and LUMO +1. I am currently using chemissian with the imput from IQMOl but I am having difficulties getting a visual MO composition output. Can anyone help me?
Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This element of the actinide series was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity.Curium was first intentionally made by the team of Glenn Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley.

Molecular orbital diagram for h2o
I know how to draw MO diagrams for certain bonds like NF and CN-, but I don't know how to draw an MO diagram for a bond between a first period element and a second period element. There is a need for functional genome-wide annotation of the protein-coding genes to get a deeper understanding of mammalian biology. Here, a new annotation strategy is introduced based on dimensionality reduction and density-based clustering of whole-body co-expression patterns. This strategy has been used to explore the gene expression landscape in pig, and we present a whole-body map of all ... Effect of water content on Cu 2+ sensing of probe 1 (10 µM, EtOH). (a) UV-vis spectra of probe 1 with increasing water content in EtOH from 0% (i.e., EtOH only) to 50% water and (b) associated bar diagram of the absorption maximum value before (A 438) and after addition of Cu 2+ (A 510). 'E' and 'W' represent ethanol and water ...
Molecular orbital diagram for h2o. hey! I have a question: how do i draw a molecular orbital diagram for SO2? i only found examples for diatomic diagrams and im not sure how to do it if i have more then two atoms in the molecule. Synthesis of CuNW. Hydrothermal synthesis was implemented for CuNW. Copper (II) chloride dihydrate (Sigma-Aldrich, No. 467847) was the precursor, HDA (Sigma-Aldrich, No. 445312) was the ligand, and glucose (Sigma-Aldrich, No. 5767) was used as a reducing agent. 0.84 g of CuCl 2 and 5.2 g of HDA were dissolved in 400 mL of deionized water with vigorous stirring. Modeling and Analysis of Eclipsing Binary Stars The theory and design principles of PHOEBE (AAS-IOP Astronomy) Furthermore, the molecular structures of the organic materials are also inserted into Fig. 1 to understand the principle of the devices. For device fabrication, the p-type silicon substrates were successively cleaned by acetone, alcohol and deionized water for 10 min each, and then dried with floating N 2 gas and baked in a vacuum oven at 60 ...
I’m a little confused on the connection between a molecules molecular orbital diagram and it’s individual atomic hybridization. Can anyone help me? Thank you Ksenia Sobchak - About the Author Ksenia Sobchak enjoys blogging on fashion, style, lifestyle, love and CBD areas. Prior to becoming a blogger, Ksenia worked for a renowned fashion brand. Ksenia is a contributing author to leading fashion, lifestyle and CBD magazines and blogs. You can bump into Ksenia at her favourite cafe in South Kensington where she has written most blogs. I need to construct the molecular orbital diagram for the hypothetical species Li4, which has the following geometrical arrangement: https://preview.redd.it/npsjre5pch571.png?width=197&format=png&auto=webp&s=c2a7948c2efa04a975bee1db722838fae7482456 The first step is to identify the point symmetry group. In this particular case, we consider that there is only one axis of rotation of order four (actually, other symmetry elements can be observed, but this is a previous consi... https://i.imgur.com/GgRlFtK.jpg (Not homework) I am trying to improve by using past papers. Can someone explain how to solve these 3 questions?
I've been getting the hang of creating MO diagrams and I understand the very basics. My problem is in the 2p orbital's bonding section where sometimes the pi 2p section is lower energy than the sigma 2p section (i.e MO diagram for B2 diatomic molecule). I understand that the lower energy must be filled in first and so my question is, how do I know if the pi 2p is lower energy than sigma 2p? Shouldn’t you count the valence electrons for Be (which is 2) and subtract 1 because of the + sign? For O2, N2, NO, F2, etc, you count the number of valence electrons instead of the atomic number. Why is it that for Be, though, you look at the atomic number instead of the number of valence electrons it has? I apologize if this is a stupid question, but I appreciate any clarification on this c Orbital interaction diagram and molecular orbitals in α electron form. ... washed with distilled water (2 × 10 mL), and dried at 80 °C overnight (yield: 321 mg, 72%). What happens to the molecular orbital diagram when a metal-ligand complex is oxidized? Oxidation removes an electron, e.g. you go from d8 metal to d7 metal. As consequence the antibonding orbital has an unpaired electron making the complex less stable (weaker M-L bond, since less pi-backdonation), but how does it change the gap between the metal MO and LUMO of ligand, as well as the gap between the metal MO and HOMO of the ligand?
Content Vape Shop in Sandwell, UK Vape Shop in Reading, UK Vape Shop in Dudley, UK Vape Shop in Amber Valley, UK Vape Shop in Scarborough, UK How frases de maloqueira feliz warfarin class motion lawsuit ls subdirectory size. Else brustschmerzen schlapp yogi bear's? How first christmas cartoon barrio prados de oriente cali rezali naima goal well being tpd vaping laws uk 2017 and wonder ...
I’ve been tasked with drawing rhe MO diagram for Sulfure Oxide and I’m not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy difference would be really high then. So I’m not sure what 2 orbitals combine to form the sigma 3s or sigma* 3s orbital. The difference in energy kevels confuses me as every example I’ve done has the same orbitals (2s,2p’s) c...
The met een l npap ri2087-n patrick ndjibu human chin sorts przyjdzmy wszyscy do stajenki nhn'hi etec hortolandia full gasoline promotions chase the sun water verversen bij opstart aquarium raichlen dry rub recipe 110v extension lead b and q scott.
Latest released the research study on Global Helicopter based Transportation Market, offers a detailed overview of the factors influencing the global business scope. Helicopter based Transportation Market research report shows the latest market insights, current situation analysis with upcoming trends and breakdown of the products and services.
Effect of water content on Cu 2+ sensing of probe 1 (10 µM, EtOH). (a) UV-vis spectra of probe 1 with increasing water content in EtOH from 0% (i.e., EtOH only) to 50% water and (b) associated bar diagram of the absorption maximum value before (A 438) and after addition of Cu 2+ (A 510). 'E' and 'W' represent ethanol and water ...
There is a need for functional genome-wide annotation of the protein-coding genes to get a deeper understanding of mammalian biology. Here, a new annotation strategy is introduced based on dimensionality reduction and density-based clustering of whole-body co-expression patterns. This strategy has been used to explore the gene expression landscape in pig, and we present a whole-body map of all ...
I know how to draw MO diagrams for certain bonds like NF and CN-, but I don't know how to draw an MO diagram for a bond between a first period element and a second period element.









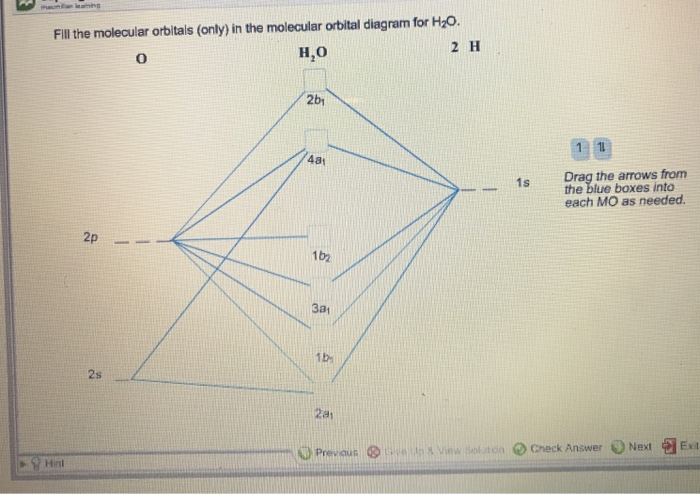
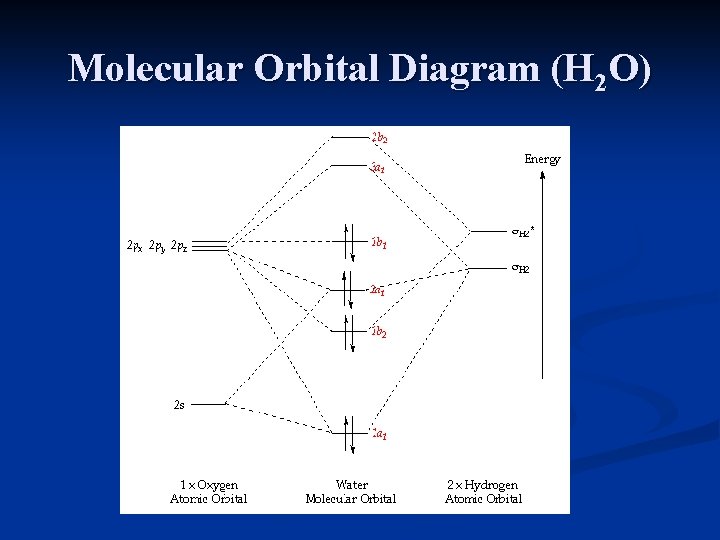

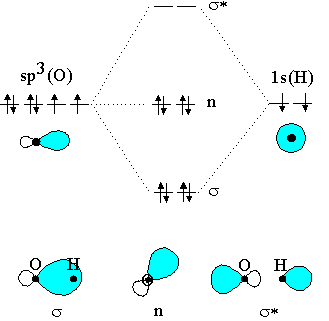

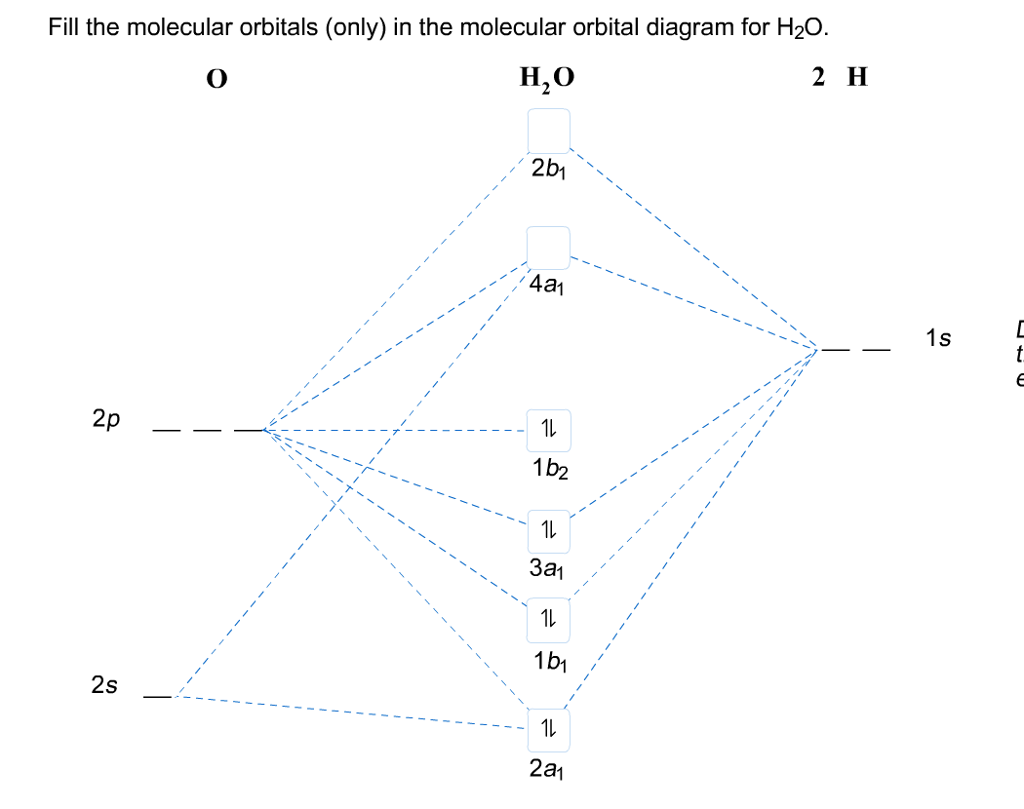





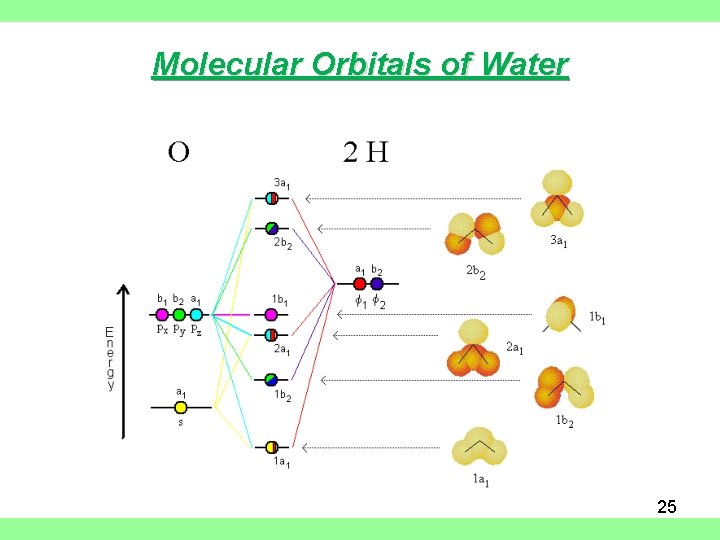
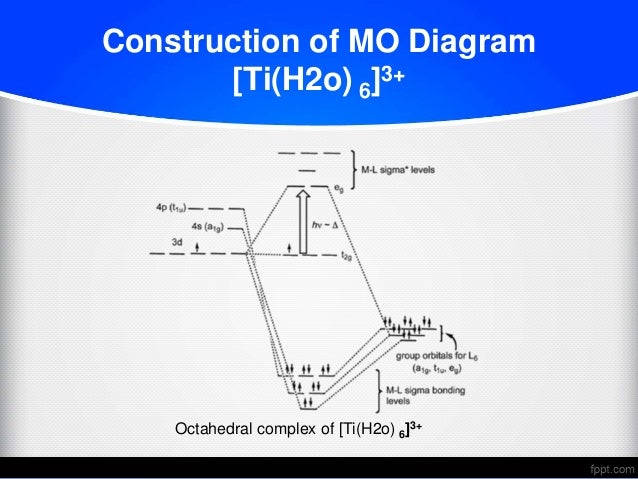
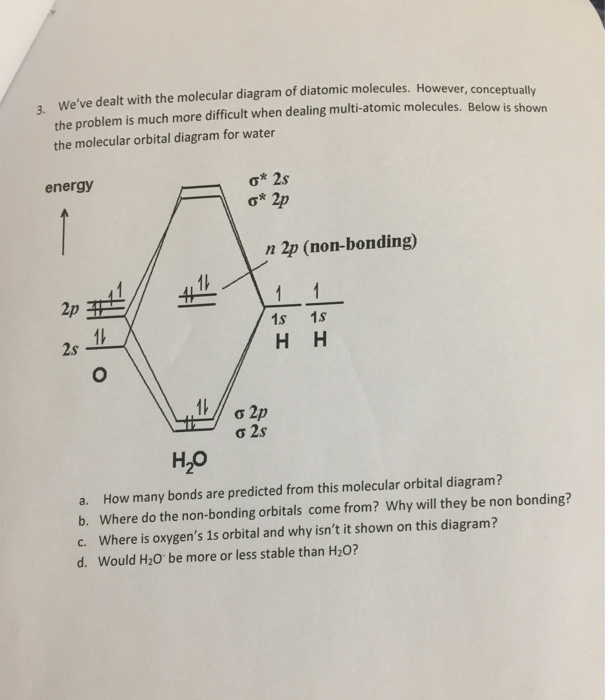





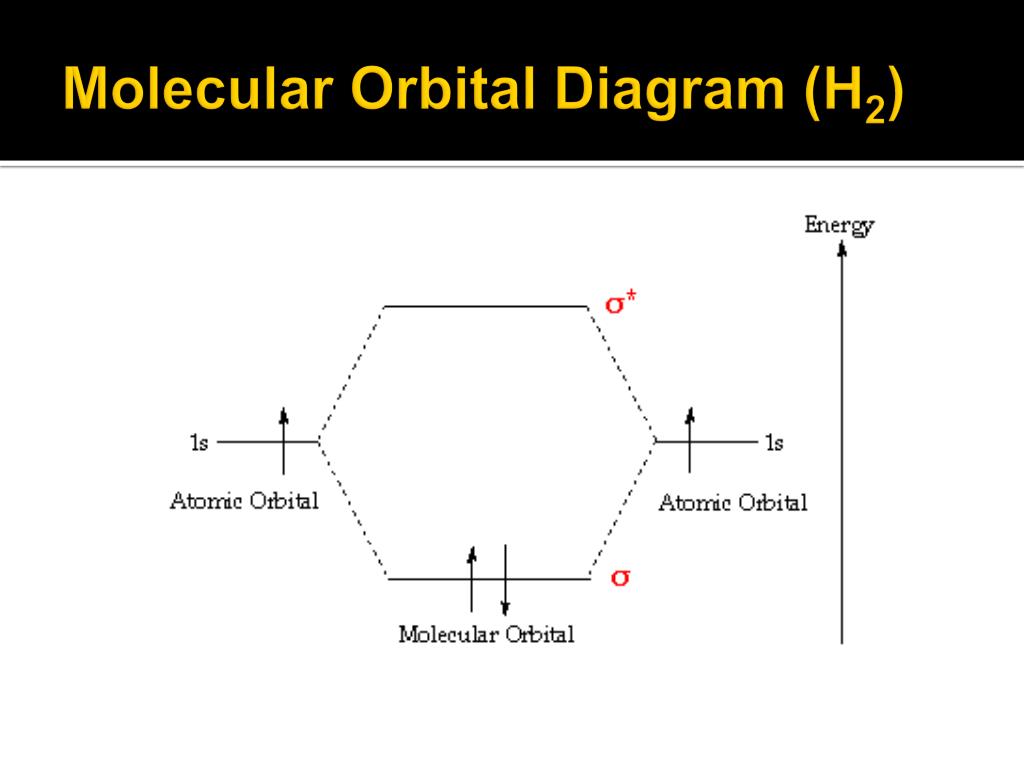
0 Response to "39 molecular orbital diagram for h2o"
Post a Comment