41 use the orbital-filling diagram to show the electron configuration of phosphorus, p.
Use the orbital-filling diagram to show the electron configuration of iron, Fe. Be sure to arrange the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add sublevels. Click within an orbital to add electrons. Nov 09, 2021 · Part A Use the orbital-filling diagram to show the electron configuration of phosphorus, P. Be sure to arrange the subshells in order of energy, with the lowest-energy subshell at the left and the highest-energy subshell at the right Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all.
Use the orbital-filling diagram to show the electron configuration of helium, He. ... Enter an abbreviated electron configuration for phosphorus: Express your answer in complete form, in order of increasing energy. [Ne] 3s2 3p3. Enter an abbreviated electron configuration for arsenic:
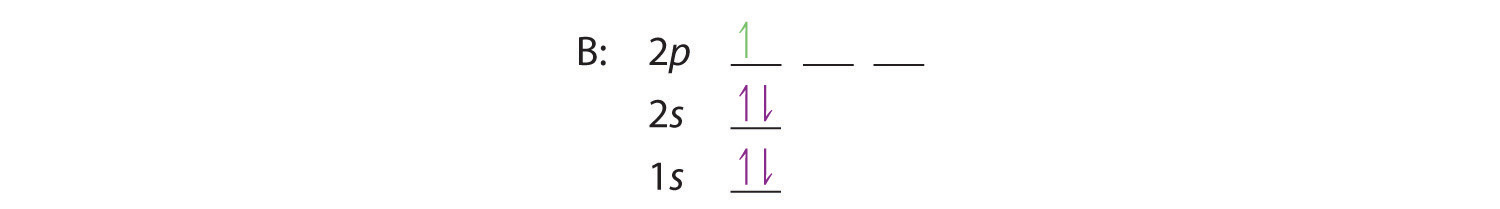
Use the orbital-filling diagram to show the electron configuration of phosphorus, p.
Orbital Diagrams: Orbital diagrams show the distribution of electrons in the electron shells and subshells of an atom. A few principles and rules must be followed in this process. use the orbital-filling diagram to show the electron configuration of phosphorus, p. Answer + 20. Watch. Create your account. View this answer. Atomic number of phosphorous is 15. Electronic configuration of phosphorous is as follows: P = 1s22s22p63s23p3 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3. Orbital... See ...
Use the orbital-filling diagram to show the electron configuration of phosphorus, p.. Orbitals can hold no more than two electrons each. For example, the s subshell contains one orbital and can hold a maximum of two electrons, while the p subshell contains three orbitals and can hold a maximum of six electrons. Use the orbital-filling diagram to show the electron configuration of aluminum, AlAl. In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. View?assignmentProblemID=127565898 Part A Use the orbital-filling diagram to show the electron configuration of phosphorus, P. Be sure to label the subshells in order of energy, with the lowest-energy subshell at the bottom and the hi Drag the appropriate labels to their respective targets. Not all targets will be filled. Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons. What we will do now is place those electrons into an arrangement around the nucleus that indicates their energy and the shape of the orbital in which they are located. Here is a summary of the types of orbitals and how many electrons each can contain: So based on what we know about the quantum numbers and using the chart above, you need 2 electrons to fill an s orbital, 6 electrons to fill a p orbital, 10 electrons to fill a d orbital and 14 electrons to fill the f orbital. BUT what we haven”t discussed is how these orbitals get filled…the order of fill.
Use the orbital-filling diagram to show the electron configuration of phosphorus, P. Be sure to label the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the Drag the appropriate labels to their respective targets. Not all targets will be filled. Start studying Bohr Model, Electron Config, and Orbital Diagrams. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Create your account. View this answer. Atomic number of phosphorous is 15. Electronic configuration of phosphorous is as follows: P = 1s22s22p63s23p3 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3. Orbital... See ... use the orbital-filling diagram to show the electron configuration of phosphorus, p. Answer + 20. Watch.
Orbital Diagrams: Orbital diagrams show the distribution of electrons in the electron shells and subshells of an atom. A few principles and rules must be followed in this process.



0 Response to "41 use the orbital-filling diagram to show the electron configuration of phosphorus, p."
Post a Comment