39 silver copper phase diagram
Teach Yourself Phase Diagrams A.4 HRS 03/11/2009 and Phase Transformations PART 1: Key terminology Alloys and Components DEF.A metallic alloy is a mixture of a metal with other metals or non-metals. Ceramics too can be mixed
Lecture Notes for MSE 2090-1 · University of Virginia, Department of Materials Science and Engineering MSE 2090: Introduction to the Science and Engineering of Materials Fall 2010 MSE 2090 - Section 1, Monday and Wednesday, 08:30 - 9:45 am, Olsson Hall 009 · Notes in pdf format Notes in pdf ...
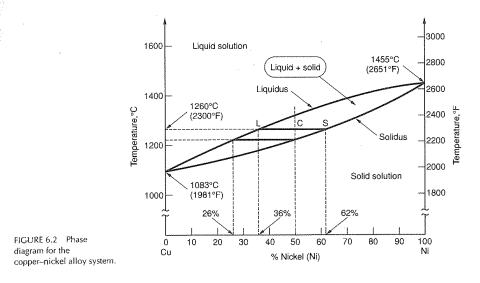
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
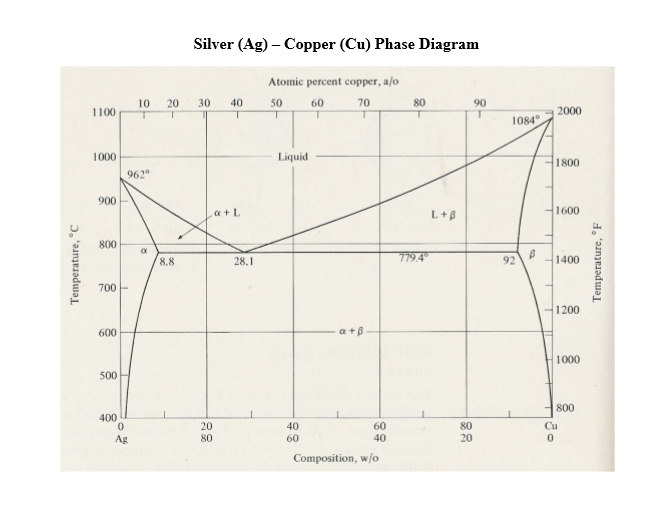
Silver copper phase diagram
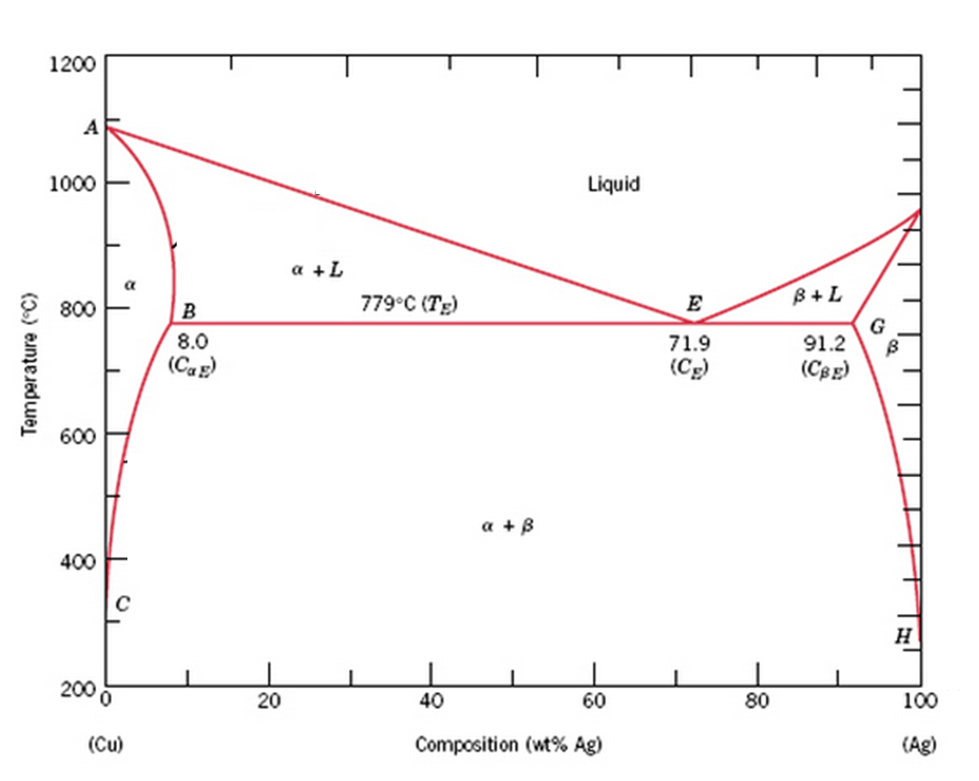
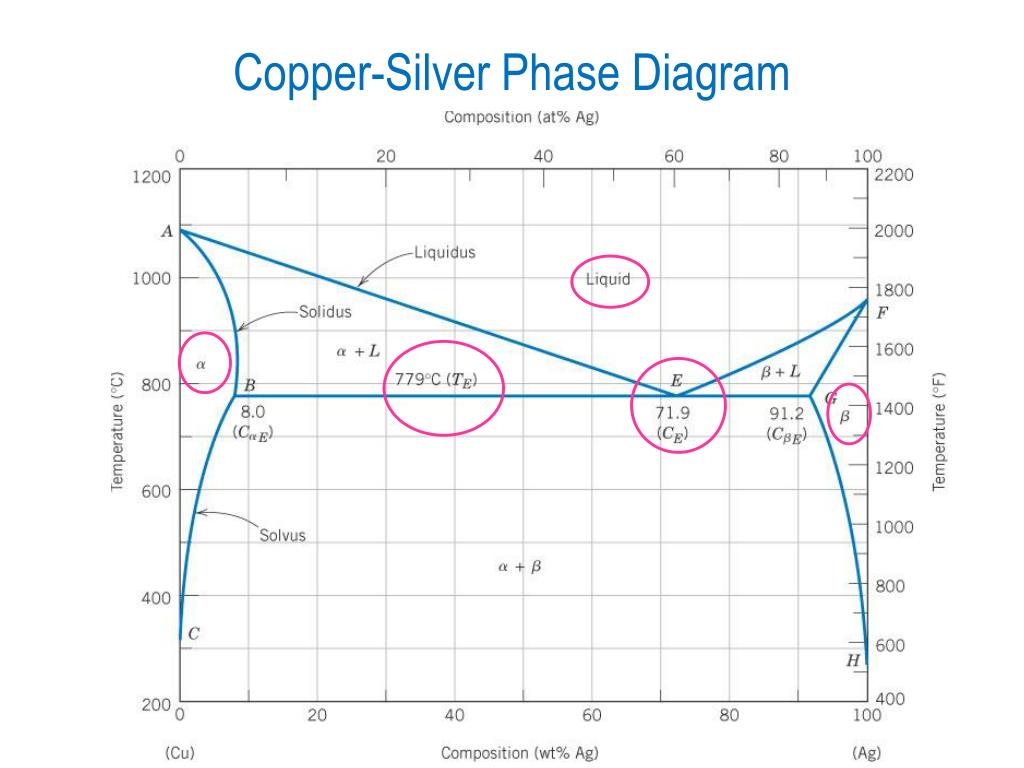
Below is a Phase diagram for silver and copper. It has a lot more going on in it than the gold silver one we were looking at before. Silver and copper are not 100% mutually soluble; they actually form a duplex alloy where there are two separate crystal structures formed by alloying silver and copper.
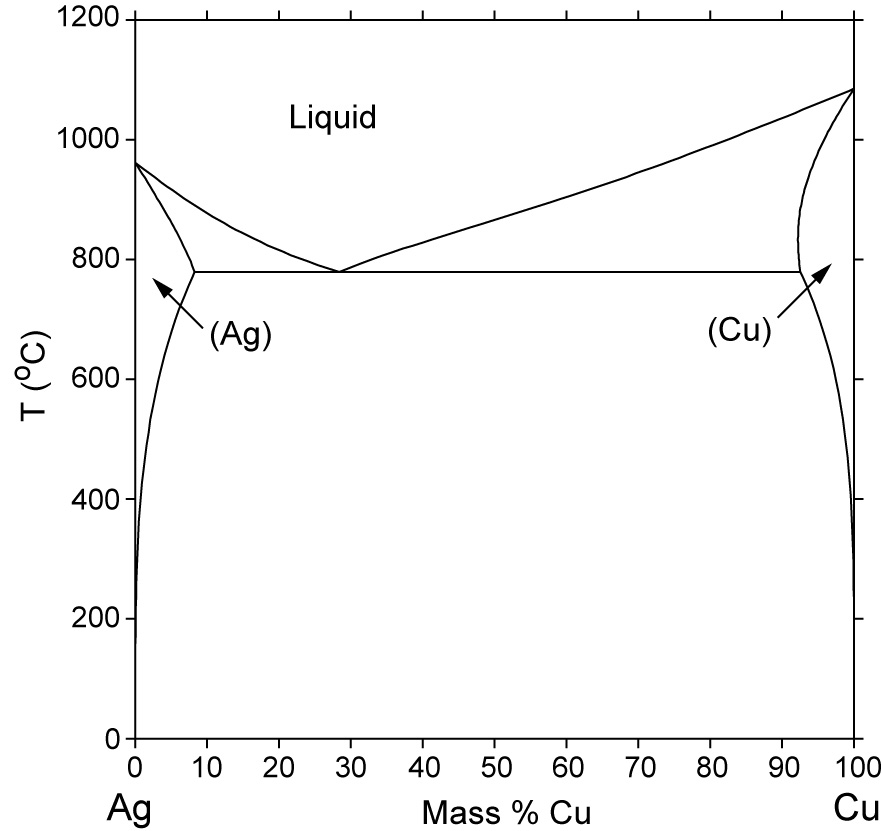
A phase diagrams show what phases exist at equilibrium and what phase transformations we can expect when we change one of the parameters of the system. Real materials are almost always mixtures of different elements rather than pure substances: in addition to T and ... Copper - Silver phase diagram liquid
Journal of Phase Equilibria - 13Ces: P. de Cesaris, "The Ternary Alloys of Nickel, Copper and Silver,"Gass. Chim. Ital., 43(2), 365-379 (1913) in Italian.(Equi ...
Silver copper phase diagram.
This video is the first part in a series about phase diagrams. This video used the eutectic phase diagram to define terminology and phase diagram calculation...
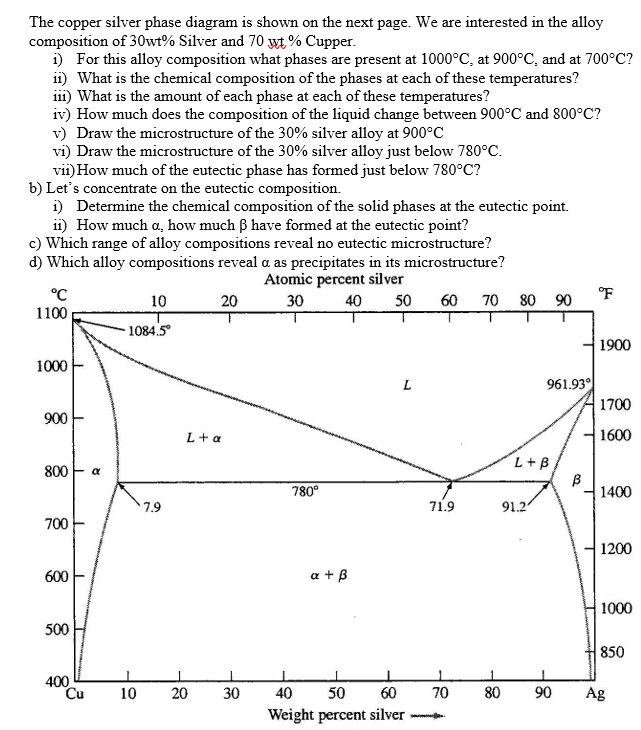
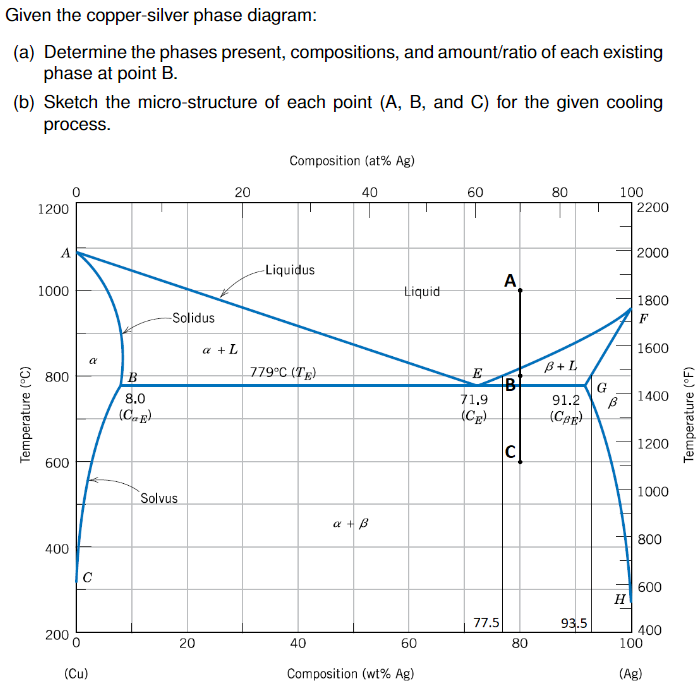
Answer to Solved questions 1-5, use the copper – silver phase diagram
Download scientific diagram | Phase diagram of Cu-Ag from publication: Fabrication, properties and microstructures of high strength and high conductivity copper-silver wires | Research results of manufacturing composite filamentary nanostructure Cu-Ag alloys with silver addition from 5 to 15% ...
Abstract. This article is a compilation of binary alloy phase diagrams for which silver (Ag) is the first named element in the binary pair. The diagrams are presented with element compositions in weight percent. The atomic percent compositions are given in a secondary scale. For each binary system, a table of crystallographic data is provided ...
Peritectic points are spots on the phase diagram at which, upon heating, a one phase solid transforms into a different solid phase mixed with a liquid. On this diagram, the peritectic is at about 38 at% platinum and 1188°C. At this point, the pure silver phase, upon heating, will decompose into a liquid mixed with the pure platinum phase.
Figure 1 shows a typical silver-copper phase diagram and it tells you a number of things. First, at all temperatures above the liquid line, any combination of silver and copper is liquid. It also identifies where the solid of any combination of silver and copper exits as one or two phases.
9.32 For a copper-silver alloy of composition 25 wt% Ag-75 wt% Cu and at 775 °C (1425 °F) do the following: ... The illustration below is the Cu-Zn phase diagram (Figure 9.19). A vertical line at a composition of 68 wt% Zn-32 wt% Cu has been drawn, and, in addition, horizontal arrows at the four temperatures called for in the ...
Download scientific diagram | Silver/copper phase diagram demonstrating that copper does not entirely dissolve in the sterling alloy, marked by the red line ...
Phase Diagrams for Lead-Free Solder Alloys Ursula R. Kattner ... silver and copper. (The elemental symbol ... Substrate materials may consist of copper, copper that has been coated or plated with tin-lead or tin-bismuth solders, nickel-tin, nickel-gold, or nickel-platinum alloys. Knowledge of the phase equilibria
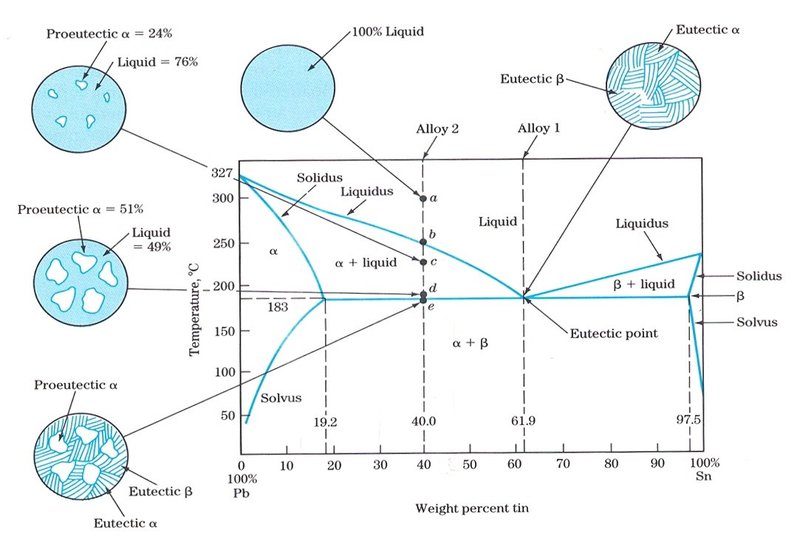
Pb-Sn Phase Diagram Liquidus Solidus Solidus Solidus Solvus Solvus · 28. 28 Solidification of Eutectic Mixtures • Mixtures of some metals, such as copper & nickel, are completely soluble in both liquid and solid states for all concentrations of both metals.
The binary phase diagram shown for the copper-nickel alloy indicates that these materials can form both liquid and solid solutions over the full range of composition from Cu to Ni. Above 1728 K, the melting point of pure Ni the alloys ar in the liquid phase. Between 1728 K and 1357 K (the melting point of Cu) the alloys can be either solid or ...
Aluminum-Copper Phase Diagram Another commonly used phase diagram is the aluminum-copper phase diagram, which is useful for understanding precipitation strengthening in Al-Cu alloys. The amount of copper present in an alloy is plotted on the x-axis. The phase fields of interest are the Al, θ, and Al+θ phase fields on the left hand side.
A region of the copper-zinc phase diagram that has been enlarged to show eutectoid and peritectic invariant points , C, 74 wt% Zn) and P (598 C, 78.6 wt% Zn), respectively. Figure by MIT OCW. Note that each single-phase field is separated from other single-phase fields by a two-phase field. Lecture 19 – Binary phase diagrams 6 of 16 11/23/05
A fourth century BCE silver jewellery collection, which is part of two hoards of Samarian coins (the Samaria and Nablus Hoards), was studied by non-destructive ...
The liquidus lines on a phase diagram is the locus of all system states that represent the boundary between a single liquid phase and the two phase (liquid + solid) zones on the diagram. For the copper-silver binary phase diagram shown a liquidus line exists between the liquid phase and (a +L), and also between the liquid phase and (b + L).
February 1, 2007 - Journal of Phase Equilibria volume 14, pages62–75(1993)Cite this article ... This is a preview of subscription content, log in to check access. ... W.C. Roberts,“On the Liquidation and Density of Certain Alloys of Silver and Copper,”Proc R. Soc. (London), 23, 481–495 (1875). (Equi Diagram; ...
Copper Silver Phase Diagram Phase Equilibria In The Agcllncl3 Ln Ce Nd Sm Gd Binary. Copper Silver Phase Diagram Materials Alloys Sterling Silver Silver Is Commonly Used In. Copper Silver Phase Diagram Electrodeposition Of Porous Silver Films On Blanket And Patterned. Copper Silver Phase Diagram Phase Diagram Solver Wiring Diagram Review
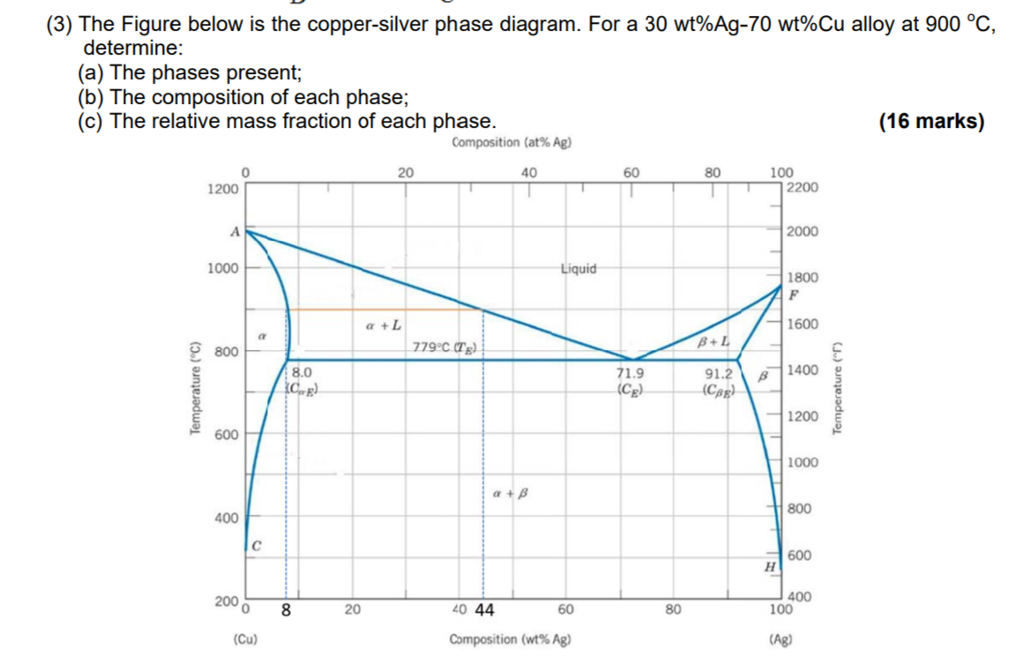
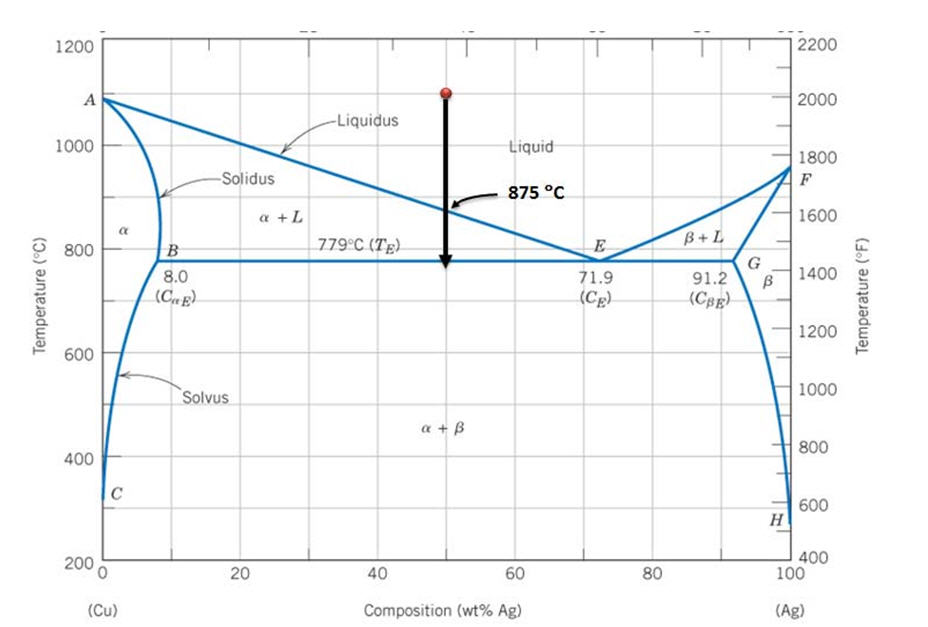
In an experiment, one selected two samples of copper-silver alloy. One sample has 40 wt% of silver and 60wt% of copper and the other has 71.9 wt% silver and 28.1wt% copper. He performed the following processes: 1). Heat the two materials to 1000 o C, keep at the temperature for long enough time. 2). Drop the temperature to 850 o C and keep at this temperature for long enough time to reach to ...
Download scientific diagram | Silver–copper phase diagram. from publication: Direct method for determining the segregation in silver-copper solid solutions not prone to brittle breakage of grain ...
November 18, 2013 - just keep some basic in mind, its give u enough information about this topic.
Phase diagram is a graphical representation of all the equilibrium phases as a function of temperature, pressure, ... Copper - Silver phase diagram . Binary Eutectic Systems • Three single phase regions (α - solid solution of Ag in Cu matrix, β = solid solution of Cu in
The copper - zinc phase diagram is a bit simpler than the copper - tin phase diagram but still complex enough. There are all kinds of brass' but typically we are at the copper-rich side. Of course, if we want to look at all copper alloys, we would need a bunch of more binary phase diagrams, in particular for the elements arsenic (As), antimony, (Sb), silver (Ag), and lead (Pb) since these are ...
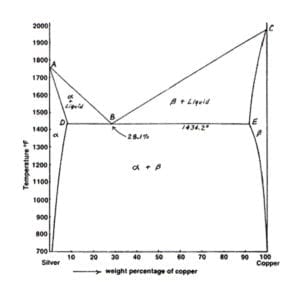
Download scientific diagram | Cu-Ag Phase Diagram. The eutectic composition is 28.1 wt% Cu-71.9 wt% Ag and the solid solubility limit of silver in copper is 8 wt% Ag (after Hansen and Anderko 1958: 18) from publication: An investigation of the mechanical and physical properties of copper-silver ...
There’s a silver-copper phase diagram at The largest area shows where the alloys are solid, and the uppermost area shows where they are liquid. The areas between represent semi-solid states. What do the remain two areas (on the left and right edges) represent? About the semi-solid states: Is this the cause of the surface
Ask any question and get an answer from our subject experts in as little as 2 hours.
A WPI education has never been more relevant than it is today, because the demand for innovative thinkers who can solve problems on a global scale has never been greater.
The copper-phosphorus binary phase diagram. The following slides show the microstructure of hypoeutectic, eutectic and hypereutectic compositions at 4.5, 8.4 and 10.5% P, respectively. ... Microstructure of wrought fine silver (left) and wrought sterling silver (right)
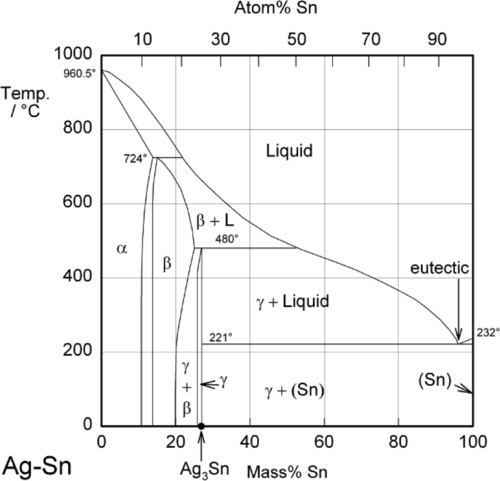
Phase diagram investigation of the Cu-Sn system was carried out on twenty Cu-rich samples by thermal analysis (DTA), metallographic methods (EPMA/SEM-EDX) and crystallographic analysis (powder XRD, high temperature powder XRD). One main issue in this work was to investigate the high temperature phases beta (W-type) and gamma (BiF 3 -type) and ...
Welcome to Materials Science · This course provides experiences in exploring materials through self-directed, hands-on projects. I hope the course environment works well for you, and I hope that you find your time in Materials Science and Solid State Chemistry interesting and enjoyable.
The silver/copper binary phase diagram is shown below. The composition of Ag-Cu alloy that will be completely melted at the lowest temperature is most nearly பால COMPOSITIONS BY TXT Hint: The eutectic composition is the point at which the liquid phase transforms directly to two solid phases.
Question: Problem 2 (0.5 pt max). The copper-silver (Cu-Ag) phase diagram is shown in the figure below. The eutectic temperature is T_E = 779°C, and the liquid contains C_E = 71.9 wt.% of silver at T_E . Phase ? is the copper-rich solid solution, and phase ? is the silver-rich solid solution.
Below is shown the titanium-copper phase diagram (Figure 9.37). There is one eutectic on this phase diagram, which exists at about 51 wt% Cu-49 wt% Ti and 960°C. Its reaction upon cooling is 2 L! TiCu + TiCu There is one eutectoid for this system. It exists at about 7.5 wt% Cu-92.5 wt% Ti and 790°C.
Reviewed under the auspices of the Alloy Phase Diagram International Commission. Element concentrations are presented in atomic percent. Document includes crystal data for the Silver-Copper system, allotropic transformation data, and related references.
Definitions and basic concepts Phases and microstructure Binary isomorphous systems (complete solid solubility) Binary eutectic systems Binary systems wit...
Answer to Consider the binary eutectic copper-silver phase diagram in Fig. P8.22. Make phase analyses of an 88 wt % Ag−12 wt %....
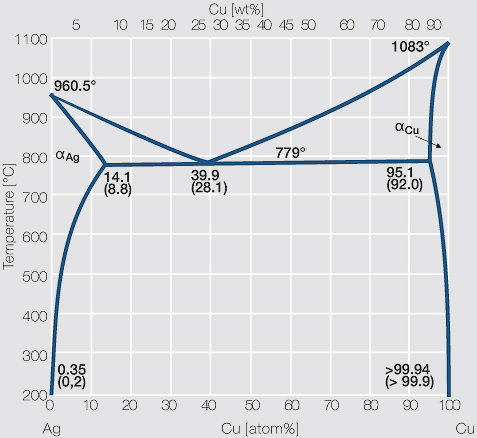
This generalization is clearly seen in the attached phase diagram for the Silver-Copper (Ag-Cu) binary system, as shown in Fig. 1, where the eutectic composition occurs at 72%Ag and 28% Cu, with a eutectic-temperature of 780°C (1435°F).
by PR Subramanian · 1993 · Cited by 206 — % Ag, resulting in a eutectic equilib- rium, as shown in the assessed phase diagram (Fig. 1). Table 1 summarizes the invariant reactions in the Ag-Cu system.
October 15, 2009 - For over 50 years, NIST has developed and distributed Standard Reference Data in Chemistry, Engineering, Fluids and Condensed Phases, Material Sciences, Mathematical and Computer Sciences and Physics..
October 4, 2021 - 404 Not Found · nginx


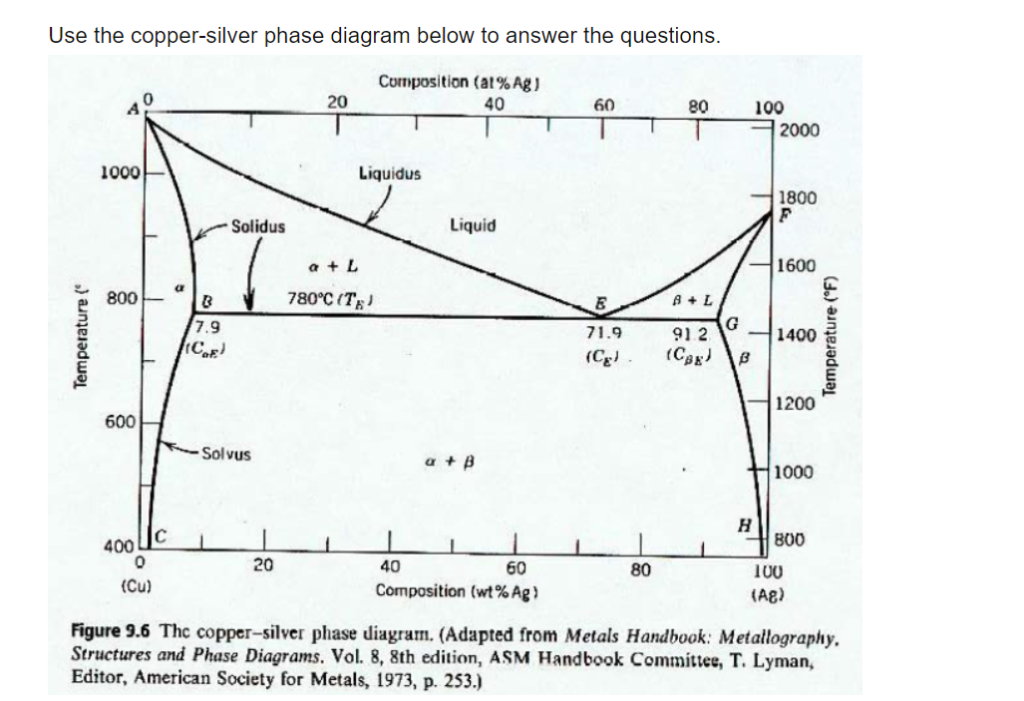









![Solved] The copper-silver phase diagram is shown in Figure 11-30 ...](https://s3.amazonaws.com/si.question.images/image/images11/814-P-M-S(1316).png)








0 Response to "39 silver copper phase diagram"
Post a Comment