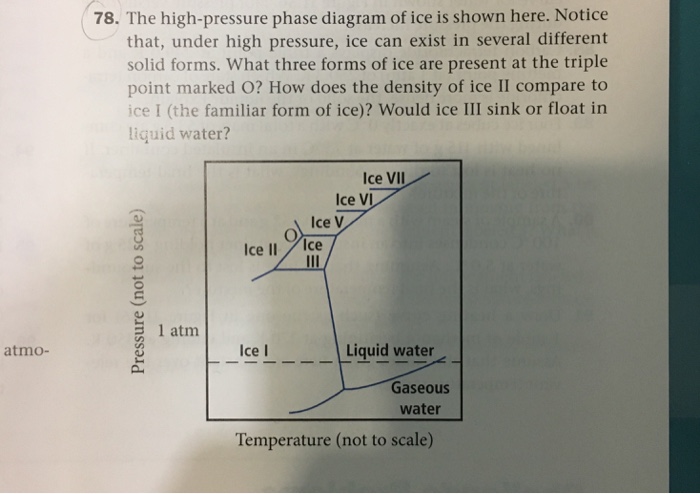
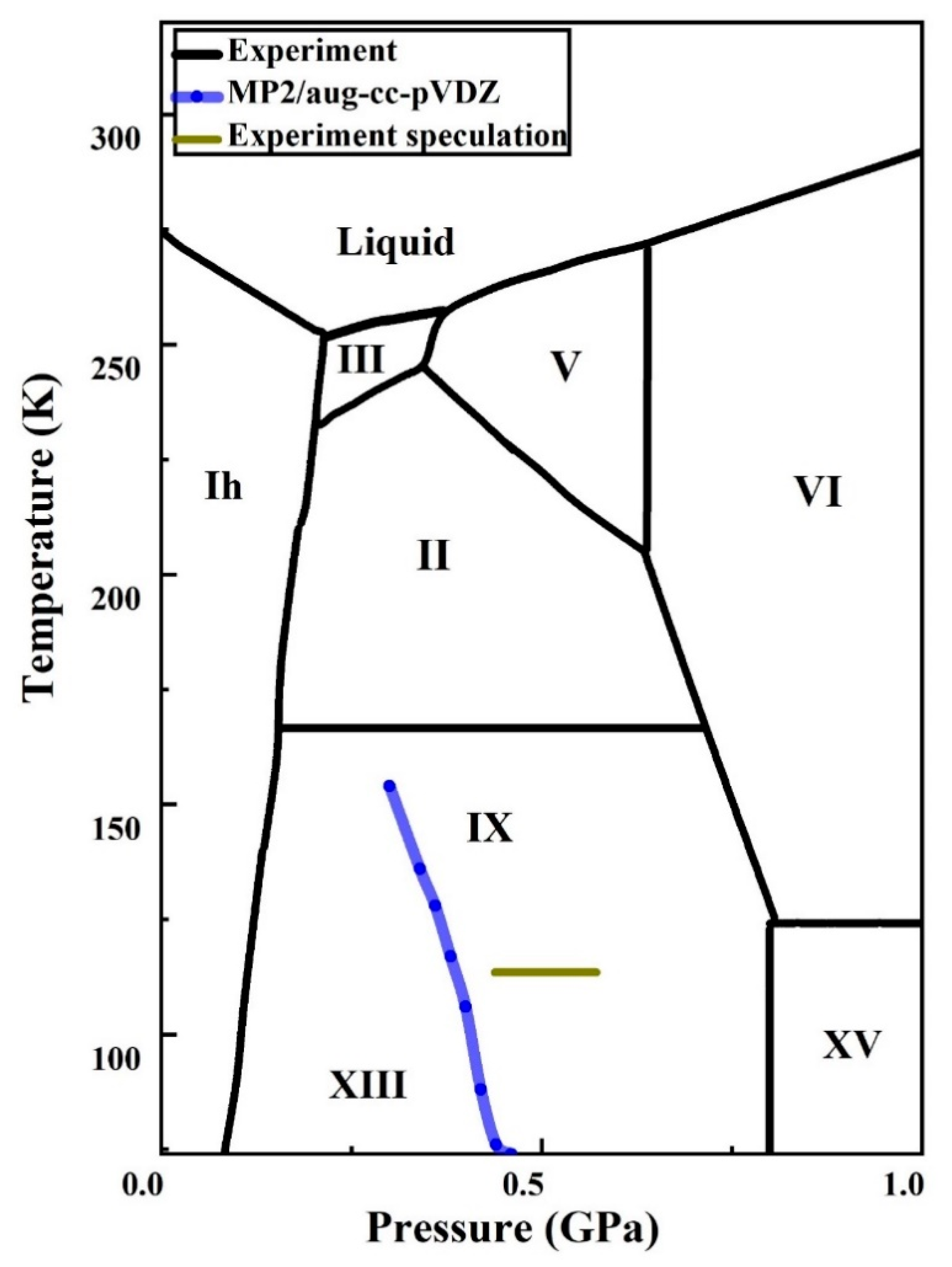
36 complete the sentences analyzing the high-pressure phase diagram of ice.
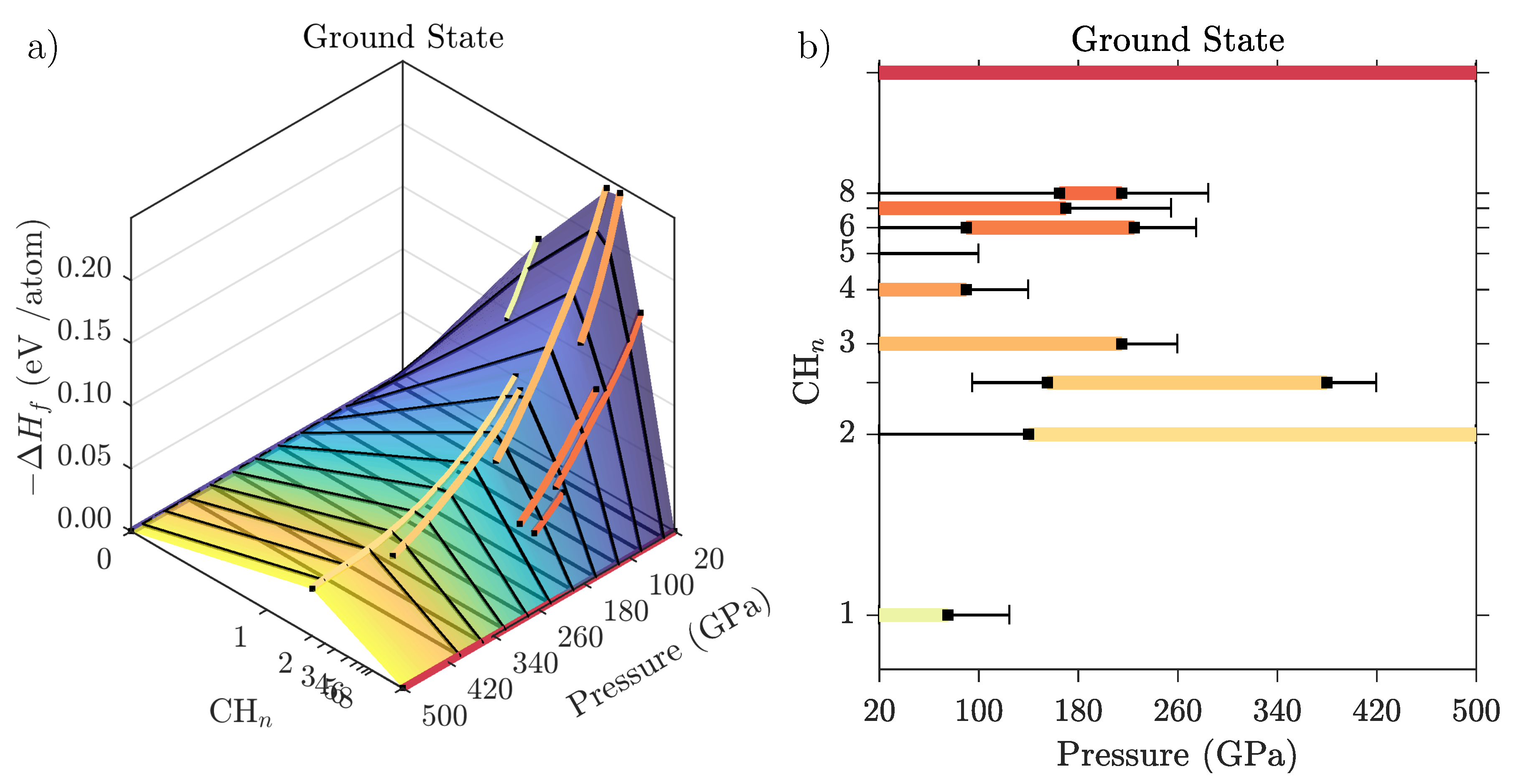
Etimologia del termine. La parola italiana libro deriva dal latino liber.Il vocabolo originariamente significava anche "corteccia", ma visto che era un materiale usato per scrivere testi (in libro scribuntur litterae, Plauto), in seguito per estensione la parola ha assunto il significato di "opera letteraria". Novel crystal structures in the C-H-O phase diagram. (a) H 2 O:2H 2, I4 1 a space group, at 100 GPa.(b) high pressure phase of oxygen, P6 3 /mmc space group, at 400 GPa.O 2 molecules are oriented ...
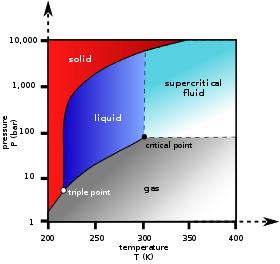
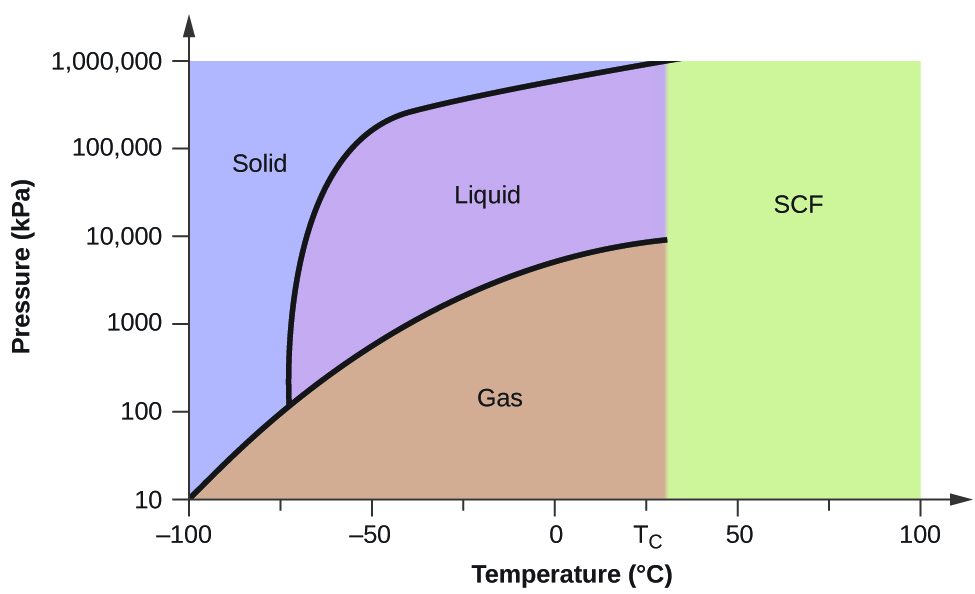
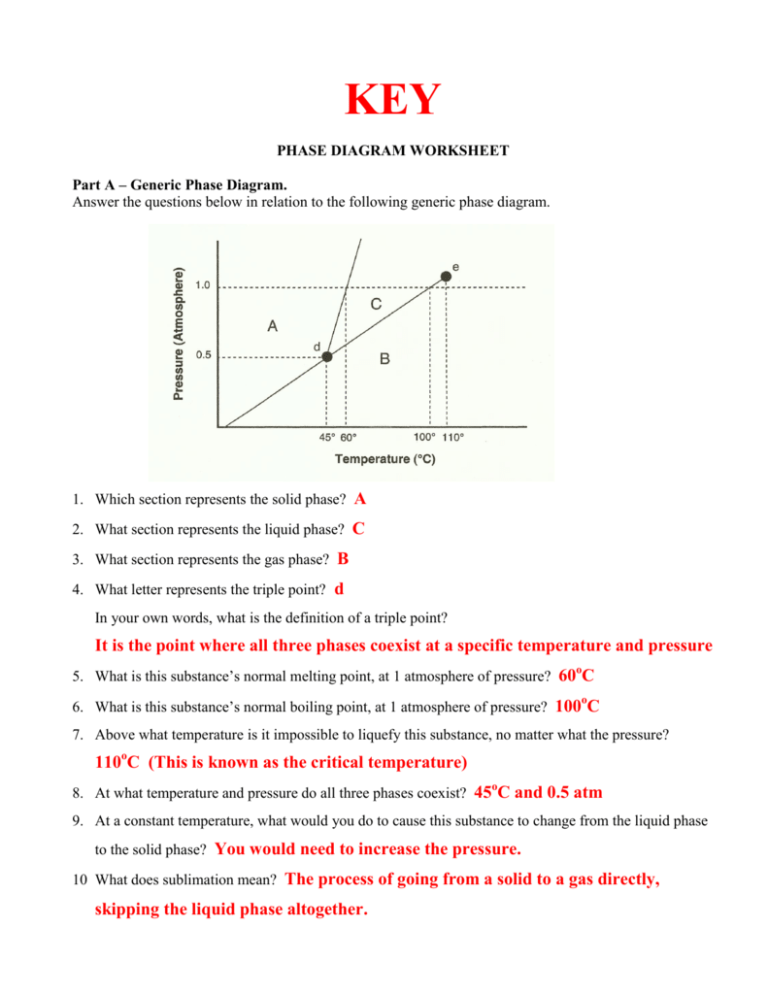
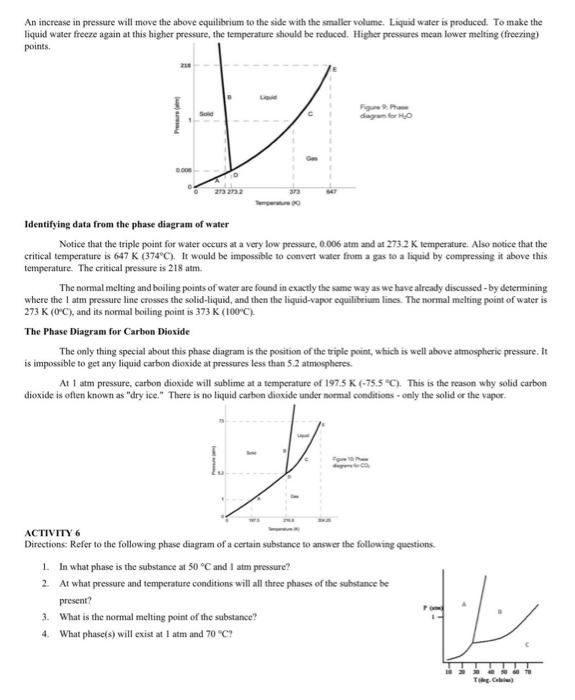
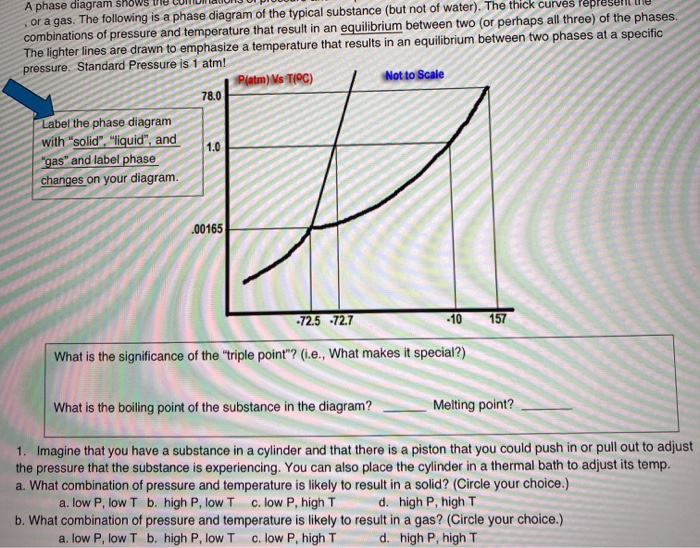
Phase Diagrams. The figure below shows an example of a phase diagram, which summarizes the effect of temperature and pressure on a substance in a closed container. Every point in this diagram represents a possible combination of temperature and pressure for the system. The diagram is divided into three areas, which represent the solid, liquid ...
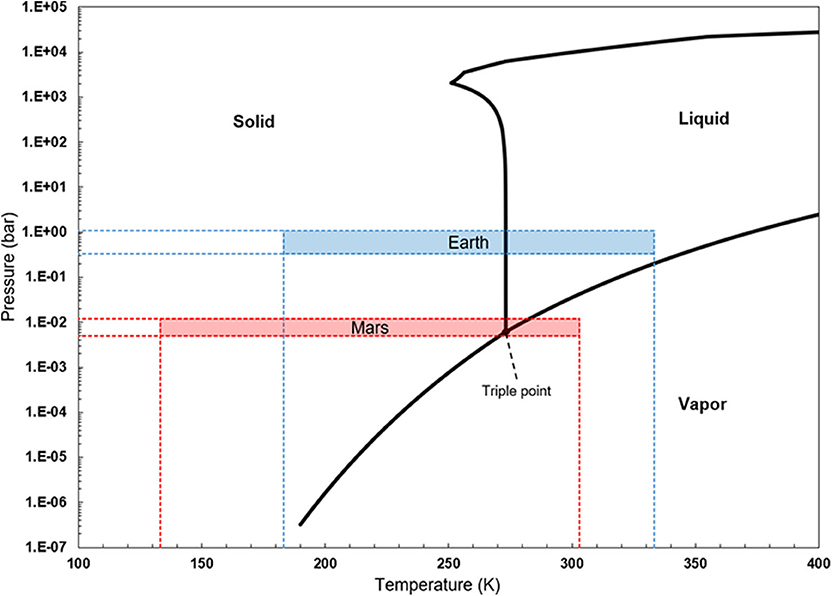
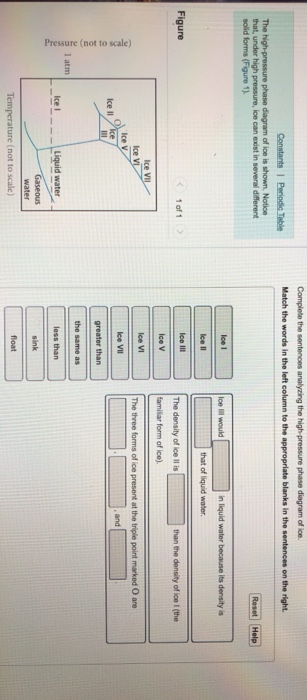
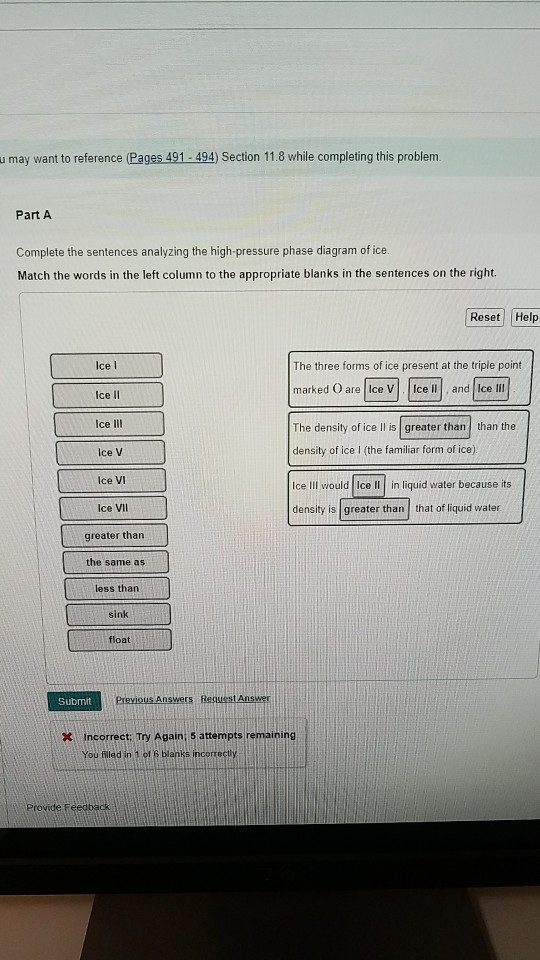
Complete the sentences analyzing the high-pressure phase diagram of ice.
Transcribed image text: Constants 1 Periodic Table hat, under high pressure, ice can exist in several different solid forms (Figure 1). lce I n liquid water because its density is Figure 1 of 1 Ice III familiar form of ice). Ice lce VI lce VIl greater than Ice Ice The three forms of ice present at the triple point marked O are and Ice lI 1 atm water float Temperature (not to scale) This chapter builds on the introduction to the arrangement of particles in materials that was covered in the chapter 'Solids, Liquids and Gases' of the Gr. 6 Matter and Materials curriculum. In Gr. 6, no distinction was made between atoms and molecules. These were grouped together and the generic term 'particle' was used to refer to these ... Sep 08, 2020 · some ice–cubes; Step 1: Fill the glass three–quarters full with water. Step 2: Add the ice–cubes to the water until the water level is close to the top. Step 3: Leves the glass until water droplets form on the outside of the glass. Explanation: The water droplets on the outside of the glass come from the air.
Complete the sentences analyzing the high-pressure phase diagram of ice.. this's Jumper eleven Section Problems seventy six. The high pressure phase diagram of Isis shown here I've done the best I could to try to represent this out of a textbook note that under high pressure ice can exist in several different ice solid forms when three wish Three forms of ice are present at the triple point marked Oh, and it's The book points to where I've indicated rate. Several aspects of the phase diagram of water at high pressure are immensely controversial: the location of the melting line 5,10,11,12,13,14,15,16,17,18 and the existence, structure, physical ... 12.4: Phase Diagrams. To understand the basics of a one-component phase diagram as a function of temperature and pressure in a closed system. To be able to identify the triple point, the critical point, and four regions: solid, liquid, gas, and a supercritical fluid. The state exhibited by a given sample of matter depends on the identity ... Our writers can complete a standard essay for you within 1-3 hours and a part of a dissertation – in 2-5 days. High Quality All the papers we deliver to clients are based on credible sources and are quality-approved by our editors.
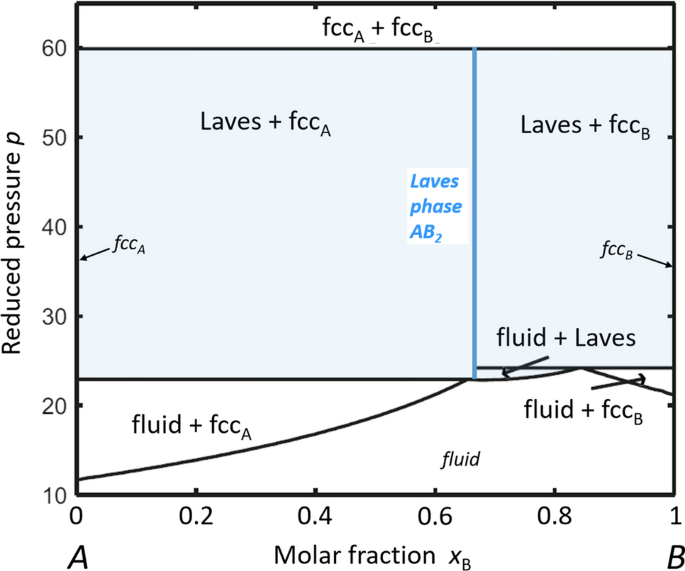
Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A typical phase diagram has pressure on the y-axis and temperature on the x-axis. As we cross the lines or curves on the phase diagram, a phase change occurs. In addition, two states of the substance coexist ... Procedure. Put about two tablespoons of water in an empty aluminum can. Set the can on a stove burner or over a flame until the the water in the can turns to steam. Use an oven mitt or tongs to carefully remove the steaming can from the heat source and immediately turn the can upside down into a bowl of cold water. At the phase transition temperature, 0 o C, all of the ice will be converted to liquid water. As soon as the phase change is complete (s ® l), addition of heat will then lead to an increase in temperature of the liquid water. The increase in temperature is, again, an increase in the KE of the system. during the phase change. Intermolecular Forces. Intermolecular Forces • Calculate the enthalpy change upon converting 1.00 mol of ice at -25 °°°°C to water vapor (steam) at 125 °°°°C under a constant pressure of 1 atm. The specific heats of ice, water, and steam are 2.09 J/g-K, 4.18 J/g-K and 1.84 J/g-K, respectively.
Ice V Ice VI Ice VII The three forms of ice present at the triple point Question : u may want to reference (Pages 491-494) Section 11.8 while completing this problem Part A Complete the sentences analyzing the high-pressure phase diagram of ice. Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash Melting Point and Freezing Point. Pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid.The transition between the solid and the liquid is so sharp for small samples of a pure substance that melting points can be measured to 0.1 o C. The melting point of solid oxygen, for example, is -218.4 o C. Academia.edu is a platform for academics to share research papers.
Some textbooks I came across, and a homework assignment I had to do several years ago, suggested that the reason we can skate on ice is the peculiar p ( T) -curve of the ice-water boundary. The reasoning is that due to the high pressure the skates put on the ice, it will melt at temperaturs below 273 K and thus provide a thin film of liquid on ...
Kshirod Kumar Dash, Sourav Chakraborty · 2021 · Technology & EngineeringLiquid water-ice I phase diagrams under high pressure: Sodium chloride and sucrose models for food systems. Biotechnology Progress, 21(2): 439–445.
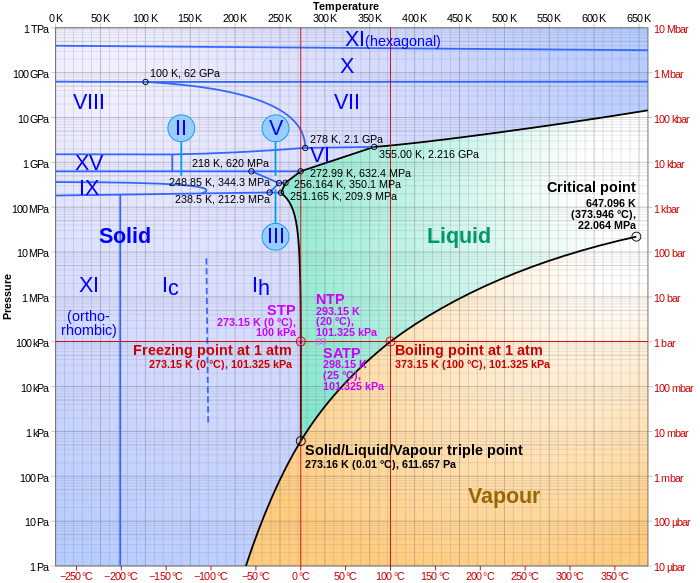
The critical point and the orange line in the ice-one phase space refer to the low-density (LDA) and high-density (HDA) forms of amorphous water (ice) . Although generally accepted, the existence of this second, if metastable, critical point is impossible to prove at the present time and is disputed by some [ 200 , 618 , 628 ].
The internal combustion engine is a heat engine.It's working principle is based on the variation of pressure and volume inside the engine's cylinders. All heat engines are characterized by a pressure-volume diagram, also known as pV diagram, which basically shows the variation of the pressure in the cylinder function of its volume, for a complete engine cycle.
(a) 1, 2, and 3 (b) 4, 5, and 6 (c) 1 and 2 only (d) 4 and 6 only (e) some other combination 11. According to the phase diagram given for Compound Y, what description is correct? (a) At the temperature and pressure at point 4, Y(g) will spontaneously convert to Y(l).(b) At 0 o C and 1200 torr, Y exists as a solid. (c) At the pressure and temperature of point 1, Y(s) will spontaneously convert ...
Example 2.4-2: Change in temperature with respect to pressure 2-24 Example 2.4-3: Estimation of thermodynamic property 2-26 Example 2.4-4: Heat required to heat a gas 2-27 Chapter 3: Phase Equilibria 3.1 Phase and Pure Substance 3-1 3.2 Phase Behavior 3-4 Example 3.2-1: Specific volume from data 3-7
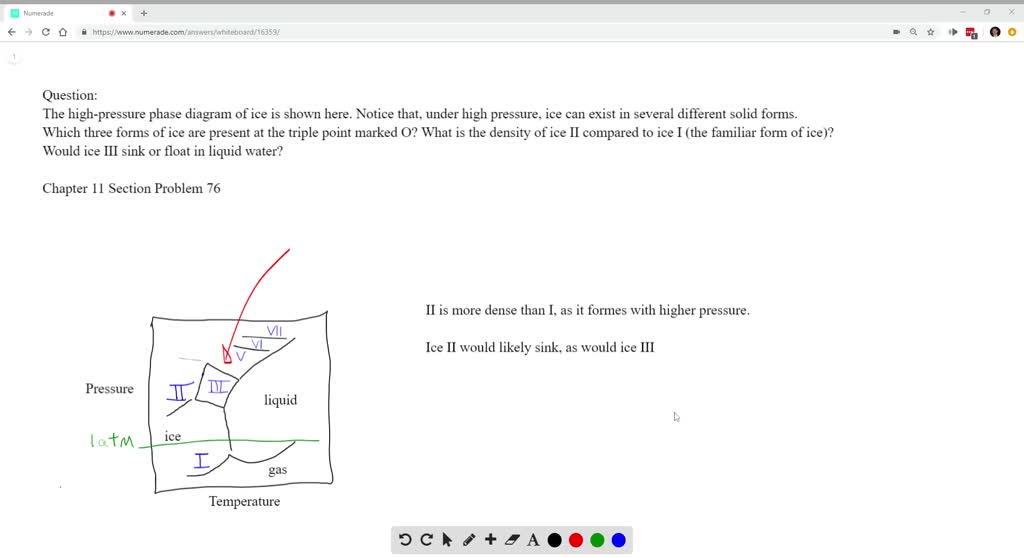
Notice that, under high pressure, ice can exist in several different solid forms.Would ice III sink or float in liquid water? FREE Expert Solution. We have to ...1 answer · Top answer: We have to determine if ice III would sink or float on liquid water.Recall that substances of lower density float over substances of highest density.A substance ...
The melting of ice under pressure is investigated with a series of first-principles molecular dynamics simulations. In particular, a two-phase approach is used to determine the melting temperature of the ice-VII phase in the range of 10-50 GPa. Our computed melting temperatures are consistent with existing diamond anvil cell experiments. We find that for pressures between 10 and 40 GPa, ice ...
The complete subject is all of the words in the subject part of a sentence. The complete predicate is all of the words in the predicate part of a sentence. COMPLETE SUBJECT COMPLETE PREDICATE The horse's black mane was blowing in the wind. The simple subject is the main person or thing in the subject part of a sentence.
A phase diagram is a temperature-pressure plot that summarizes the conditions under which a substance exists as a solid, liquid, or gas. The curves that separate the phases are known as phase boundaries. Each phase boundary represents the equilibrium between the phases on either side of the curve. Identify the components of the phase diagram of ...
Academia.edu is a platform for academics to share research papers.
Pulse is the count of surges of pressure in an artery that occur with each contraction and relaxation of the left ventricle. Compute the pulse pressure of a person with a BP of 120/90. Drag and drop the correct numbers to complete the calculation. 30 = 120 - 90.
The high-pressure phase diagram of ice is shown here. Notice that, under high pressure, ice can exist in several different solid forms. Which three forms of ice are present at the triple point marked O? What is the density of ice II compared to ice I (the familiar form of ice)? Would ice III sink or float in liquid water?
If you need help refer back to Part C - Plotted weather charts.. Worksheet 1 - extension exercise. The following diagram shows a series of plotted pressure values. Download a copy of the worksheet here.Complete the diagram by drawing isobars at intervals of 4 millibars, including 992, 996, 1000, 1004 and 1008.
We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice.". Under these conditions, water exists only as a solid (ice).
Academia.edu is a platform for academics to share research papers.
High pressure (or temperature) and low pressure (or temperature). This assumes the condenser output is saturated liquid and the boiler output is saturated vapor. Physically the high pressure is determined by the pump and the low temperature is determined by the cooling medium. b. Superheat. Three single properties.
An accurate experimental method, based on the pressurization of an ice-solution mixture, adequate to build the entire phase transition curve at constant ...
Then analyze the sign of the equation to determine whether each critical point is stable or unstable, and construct the corresponding phase diagram for the differential equation. Next, solve the differ ential equation explicitly for x(t) in terms of t. Finally, use either the exact solution or a computer-generatedslope field to sketch typical
Murthy S. Gudipati, Julie Castillo-Rogez · 2012 · Science5.5 A Few Words About Limitations After this brief overview of the DFT and the few ... Focher P, Parrinello M (1996) New high-pressure phase of ice.
Sep 08, 2020 · some ice–cubes; Step 1: Fill the glass three–quarters full with water. Step 2: Add the ice–cubes to the water until the water level is close to the top. Step 3: Leves the glass until water droplets form on the outside of the glass. Explanation: The water droplets on the outside of the glass come from the air.
This chapter builds on the introduction to the arrangement of particles in materials that was covered in the chapter 'Solids, Liquids and Gases' of the Gr. 6 Matter and Materials curriculum. In Gr. 6, no distinction was made between atoms and molecules. These were grouped together and the generic term 'particle' was used to refer to these ...
Transcribed image text: Constants 1 Periodic Table hat, under high pressure, ice can exist in several different solid forms (Figure 1). lce I n liquid water because its density is Figure 1 of 1 Ice III familiar form of ice). Ice lce VI lce VIl greater than Ice Ice The three forms of ice present at the triple point marked O are and Ice lI 1 atm water float Temperature (not to scale)











0 Response to "36 complete the sentences analyzing the high-pressure phase diagram of ice."
Post a Comment