35 construct the octahedral crystal-field splitting diagram for the metal in each species.
The adsorption energy of the Ag dimer structure is calculated to be −0.452 eV (Ag 2 (a) in Table S2 ), less stable than the adsorption energy of the isolated Ag atom by 0.242 eV. It can be supposed that the weaker adsorption of the Ag dimer on the β-Ga 2 O 3 surface results in longer Ag-O bond distances. (23) Table 2.
A detailed analysis of the electronic structure of the [(CF 3) 2 AgBr 3] 2− anion under imposed D 3h symmetry (Figure 4) 31 reveals that the MOs with major metal contribution are well below the HOMO and inverted in order with respect to the standard arrangement derived from D 3h ligand-field splitting. 32 Thus, the a 1 ′ MO with mainly d ...
Certain Tanabe-Sugano diagram s (d4, d5, d6, and d7) also have a vertical line drawn at a specific Dq/B value, which corresponds with a discontinuity in the slopes of the excited states' energy levels. This pucker in the lines occurs when the spin pairing energy, P, is equal to the ligand field splitting energy, Dq.
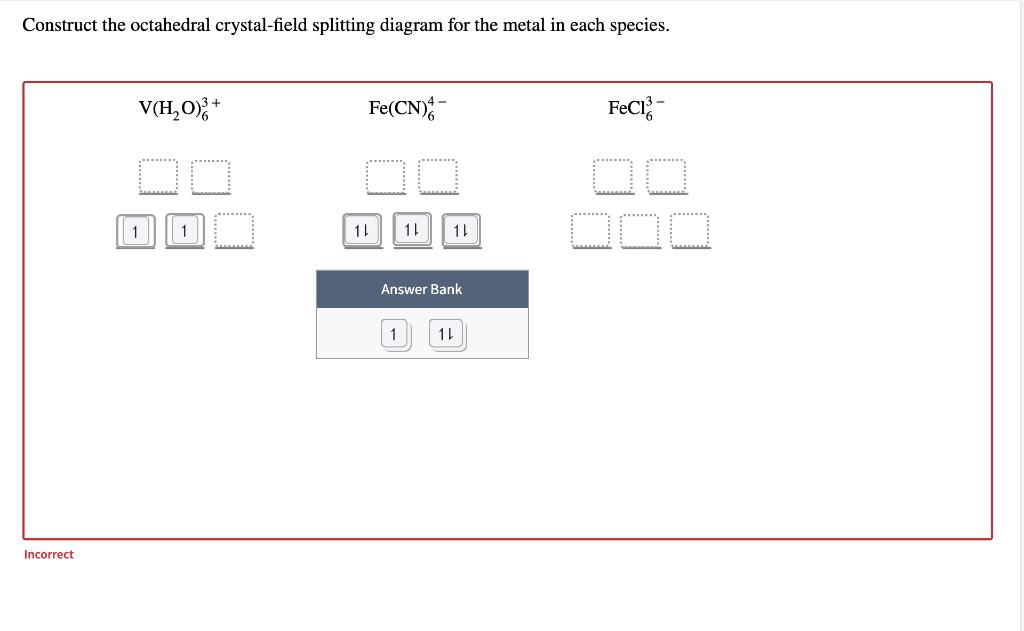
Construct the octahedral crystal-field splitting diagram for the metal in each species.
The pattern of splitting depends upon the nature of the crystal field. 56. • (a) Crystal field splitting in octahedral coordination entities: • In an octahedral coordination entity with six ligands surrounding the metal atom/ion, there will be repulsion between the electrons in metal d orbitals and the electrons (or negative charges) of the ...
The crystal field stabilization energy (CFSE) is the stability that results from placing a transition metal ion in the crystal field generated by a set of ligands. It arises due to the fact that when the d orbitals are split in a ligand field, some of them become lower in energy than before. For example, in the case of an octahedron, the t 2g
Spin-orbit effects in heavy 5d transition metal oxides, in particular, iridates, have received enormous current interest due to the prediction as well as the realization of a plethora of exotic and unconventional magnetic properties. While a bulk of these works are based on tetravalent iridates (d 5), where the counter-intuitive insulating state of the rather extended 5d orbitals are ...
Construct the octahedral crystal-field splitting diagram for the metal in each species..
Jiang, H. et al. Ultrathin Ti 3 C 2 T x (MXene) nanosheet-wrapped NiSe 2 octahedral crystal for enhanced supercapacitor performance and synergetic electrocatalytic water splitting. Nano-Micro Lett ...
Answer: I do not think I agree with the question. T₂𝘨 and e𝘨 orbitals are two sets [1] of the d subshell of atomic orbitals that arise from an isolated, gaseous transition metal [2] ion when six ligands [3] are brought in to coordinate said ion in an octahedral geometry: The process is describe...
The nature of the released metal ion could damage the cells by producing reactive oxygen species (ROS), or metal ions can help to neutralize ROS . As another study of the effects of metal ion and organic ligand on the toxicity, Ruyra, A. et al. evaluated in vitro and in vivo toxicity of different MOFs composed of various metals and ligands ...
The magnitude of crystal field stabilization energy in octahedral field depends on I the nature of the ligand Il : the charge on the metal ion III : whether the metal is in the first, second or third row of the transition elements. A. ( I, ) II, III all are correct B. I, II are correct c. ॥, Ill are correct D. III only correct: 12: 211
Metal coordination and metal activation abilities of commonly
The absorption band peaks at 627 nm were assigned to d-d transition, showing 10Dq = 15,949 cm −1 and crystal field stabilization energy (CFSE) = 0.6 × 10Dq = 114.4 kJmol −1, while the ligand-to-metal charge transfer (LMCT) of complexes displayed at 292 nm. The intense luminescence band results from the ligand-to-metal charge transfer ...
The splitting of 3d levels in an octahedral crystal field is determined by the 10Dq parameter. The Tanabe-Sugano diagrams are normally used to obtain a preliminary estimate of the spin state of a system of cobalt ions [ 1 ], which show the dependence of the energy position of the electronic levels on the crystal field value determined by the ...
(a) Spin-polarized density of states of SRO trilayer at − 0.47 % strain as computed in LDA, projected onto the octahedral crystal-field split Ru-t 2 g states. An orthorhombic distortion of RuO 6 octahedra gives rise to a large splitting of the Ru-t 2 g states. (b) Spin-polarized density of states of SRO trilayer at − 1.7 % strain.
The spin-allowed 3 A 2g (F) → 3 T 1g (F) transitions of the d 8 geometry of Ni 2+ ions in the octahedral crystal field splitting energy are occurred at 750 nm band, and the absorption band at 650 nm is attributed to the special spin-forbidden 3 A 2g (F) → 1 E g (D) transitions .
(a-b) The local coordination of transition metals, each transition-metal ion is subjected to an octahedral crystal field. (c) The simplified density of states, the nonbonding d-orbitals of the MXenes are positioned between bonding (σ) and antibonding (σ *) states of M−X and M−T bonds. (d) Occupation of the electrons on the transition ...
In the bulk, octahedral ligand field demonstrates that each metal atom is fully coordinated with six oxygen atoms (named as MO 6) and splits to higher e g and lower t 2g orbitals, as shown in Fig. 1a.
For transition metals in the octahedral crystal field, the splitting of d-orbitals results in the formation of three t 2g and two e g orbitals. Among which, only the e g orbitals have a density of states that is out of the plane (Figure S25c), which enables an overlap with the O 2p orbitals of oxygen intermediates (Figure S25d).
Question 9.16 Draw figure to show the splitting of d orbitals in an octahedral crystal field. Answer : The splitting of d orbital is shown below:- In this splitting d x 2 y 2 and d z 2 experience a rise in energy and make the eg level, while d xy , d yz and d zx experience a fall in energy and generate the t 2g level.
Answer to Write orbital diagram for Co2+. Draw The Orbital Diagram For Ion Co 2 Clutch Prep. Sigma Pi Bonding Atomic Orbital Bonding Sigma S 1) Draw the octahedral crystal field splitting diagram for each metal ion. a) V3+ b) Co2+ (high-spin) 2) The [CrCl6]3− ion has a maximum in its absorpt ion spectrum at 735 nm.
13. In an octahedral crystal field, draw the figure to show splitting of d orbitals. 14. What is linkage isomerism? Explain with an example. 15. Write briefly about the applications of coordination compounds in volumetric . analysis. 16. Classify the following ligand based on the number of donor atoms. a) NH3 b) en c) ox2- d ...
As known, for LCO, E g greatly relates to the crystal field splitting energy (Δ CF) of CoO 6 octahedron . The action of octahedral crystal field can lead to the five-fold degeneracy of Co 3+ 3d orbital broken down into a high-energy double state (e g orbital) and a low-energy triplet state (t 2g orbital), and their energy difference is Δ CF .
Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species, a) V(H_2O)_6^3+ Co(CN)_6^3- FeCl_6^3-.
The crystal field splitting in tetrahedral complex is represented in the energy level diagram as shown in Figure 16.14. Figure 16.14 Crystal Field Splitting in Tetrahedral Complex Unlike the octahedral complexes where low-spin and high-spin complexes do exist, there are only high-spin complexes known for tetrahedral complexes.
Question: Construct the octahedral crystal-field splitting diagram for the metal in each species. V ( H 2 O ) 3 + 6 V(H2O)63+ Co ( CN ) 3 − 6 Co(CN)63− Mn ...
an crystal field splitting diagrams to show orbital occupancies in both weak and strong octahedral fields, and (ii) indicate the number of unpaired electrons in each case. Label. the diagrams (iii) weak or strong field, (iv) high spin or low spin (as appropriate), (v) with the names of the d-orbitals, and (vi) with the appropriate orbital sets.
Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species, V(H_20)^3+_6 Fe(CN)^4-_6 FeCle^3-_6.
Octahedral case. Suppose a complex has an octahedral coordination sphere. Assume the six ligands all lie along the x, y and z axes. There are two d orbitals that will interact very strongly with these ligands: the d x 2-y 2, which lies directly on the x and y axes, and the d z 2, which lies directly on the z axis.Together, these two metal orbitals and the ligand orbitals that interact with ...
Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H2O)63+ Co(CN)63 - Mn(H2O)62+.
Construct a qualitative molecular orbital diagram of a square pyramidal complex using the symmetry adapted linear combination of atomic orbitals approach. Consider sigma-bonding only. Answer. Exercise 11. Strong π-donating ligands lead to a decrease of the octahedral ligand field Δo. Illustrate this by sketching the relevant part of MO ...
Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. a)V(H_2O)6^3+ b)Co(CN)6^3- c) FeCl_6^3-.
Colors of Coordination Complexes: Crystal Field Splitting. When ligands attach to a transition metal to form a coordination complex, electrons in the d orbital split into high energy and low energy orbitals. The difference in energy of the two levels is denoted as ∆, and it is a characteristic a property both of the metal and the ligands.
Question: Construct the octahedral crystal-field splitting diagram for the metal in each species. This problem has been solved! See the answerSee the ...
This is analogous to deciding whether an octahedral complex adopts a high- or low-spin configuration; where the crystal field splitting parameter \(Δ_o\) \(ΔE\) does above. Unfortunately, unlike \(Δ_o\) in octahedral complexes, there is no simple graphical way to represent \(ΔE\) on the diagram above since multiple orbitals are changed in ...
Question: Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H_2O)_6^3+ Co(CN)_6^3- Mn(H_2O)_6^2+ ...
Figure \(\PageIndex{5}\): (a) Tetraheral ligand field surrounding a central transition metal (blue sphere). (b) Splitting of the degenerate d-orbitals (without a ligand field) due to an octahedral ligand field (left diagram) and the tetrahedral field (right diagram).
Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H_2O)_6^3+ b) Fe(CN)_6^4- c) FeCl_6^3-.
Construct the octahedral crystal field splitting diagram for the metal in each species. since the oxalate ligand is fairly low in the series a weak field ligand at this point you may not have studied ligand field theory yet which explains why it is a weak ligand. cr4 mnh2o62 asked by katie on march 30 2012 chemistry based on crystal field.
Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H2O)63+ Co(CN)63 - Mn(H2O)62+.


0 Response to "35 construct the octahedral crystal-field splitting diagram for the metal in each species."
Post a Comment