39 tin electron dot diagram
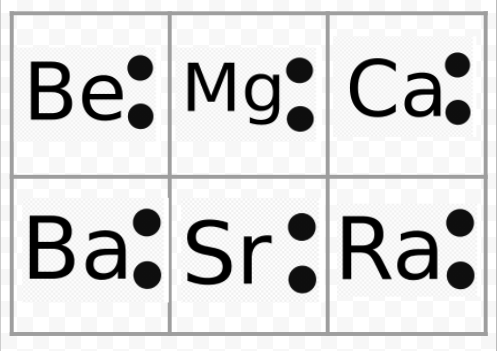
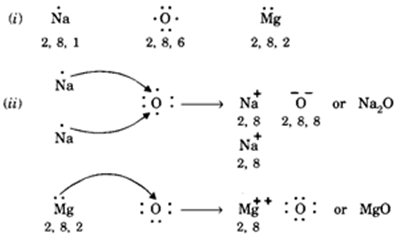
Two separate fluorine atoms have the following electron dot diagrams: Each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule: ... Predict the formula of a compound between tin and hydrogen. Astatine is a synthetic element, made one atom at a time in ... Dots placed around the chemical symbol represents electron dot structure valence electrons. Electrons are placed up to two on each side of the elemental symbol for a maximum of eight, which is the number of electrons in a packed s and p shell. The elements that have fewer than four dots in the diagrams are Strontium and Gallium.
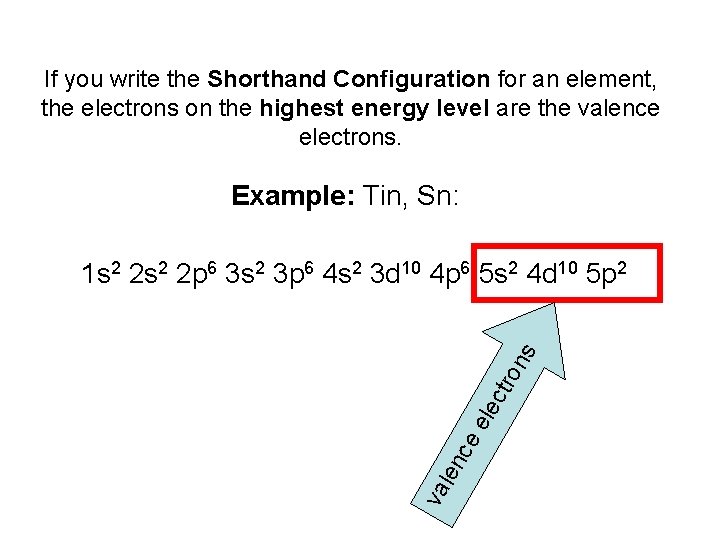
Write electron dot diagrams for the following atoms: a. silicon b. rubidium c. barium d. tin e. iodine f. arsenic . Si Rb Ba Sn I As . 4 dots, 1 on 1 dot 2 dots, 1 4 dots, 1 7 dots, 2 dots on 5 dots, 2 dots on 1 side, each side each on 2 sides on each side 3 sides, 1 dot on 1 dot on each of the ...

Tin electron dot diagram
A step-by-step explanation of how to draw the Arsenic (As) Lewis Dot Structure.For the ArsenicLewis structure use the periodic table to find the total number... c Dispersive gate charge sensing of a double quantum dot formed under P1 and P2 down to single electron occupancy using the high-impedance TiN resonator as a dispersive gate readout. The main uses of stannic chloride are as a raw material for the manufacture of other tine compounds, especially organotins. and in the surface treatmentof glass and other nonconductive materials, whereby stannic oxide is deposited from the stannic chloride solutions onto the surface giving it strength, abrasive resistance, and conductivity. It is also widely used as a catalyst in Friedel-Craft ...
Tin electron dot diagram. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ... A step-by-step explanation of how to draw the Cs Lewis Dot Structure.For the Cs structure use the periodic table to find the total number of valence electron... electrons and draw the Lewis dot structure. 1. Barium 6. Carbon. 2. Tin 7. Krypton. 3. Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) are . Neon (Ne), argon (Ar), krypton (Kr), etc., each contain eight electrons in their. Column 1A 1 valence electron The first symbol in the column is H . electrons and draw the Lewis dot structure. 1. 1. An electron dot diagram can show you that the symbols for an element surrounded by dots. Each dot stands for one valence electron.
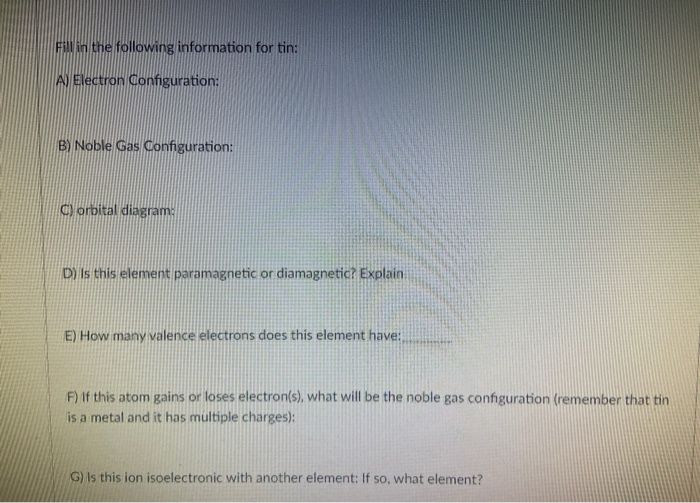
Lewis Dot Diagrams of Selected Elements. Lewis Symbols: Electron Configuration into Shells: Index Chemical concepts Chemistry of the Elements Periodic Table . HyperPhysics***** Quantum Physics : R Nave: Go Back: Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its ... 3. When writing the electron configuration of an atom, in what general order are the sublevels written? 4. How is the number of electrons in an energy sublevel indicated in an electron configuration? 5. What is the electron configuration for tin (Sn)? 6. The valence orbitals in an atom are the _____. 7. What is an electron dot structure? 8. A Lewis Dot Structure is the diagrammatic representation of the bonding between the atoms of a molecule and the lone pair of electrons present in it. It is also known as electron dot structure/ Lewis dot diagram, Lewis dot formulas, Electron dot structure, or Lewis Electron dot structure (LEDs), respectively. Which elements have fewer than four dots in their electron dot diagrams? Check all that apply. arsenic (As) strontium (Sr) krypton (Kr) gallium (Ga) tin (Sn) sulfur (S) strontium (Sr) and gallium (Ga): have fewer than four dots in their electron dot diagrams. s. Log in for more information. Question. Asked 11/15/2016 9:21:59 AM.
Which is the ending electron configuration of tin? 5p2. How many dots would appear on the Lewis electron dot diagram for an atom whose electron notation ends in 6s25d106p4? 6. Element X has an electron configuration that ends with 4p4. How many electrons would it like to gain to achieve stability? 2. Electron dot formula shows the number of valence electrons for that element with the help of dots. The valence electrons are those electrons that occupy the highest energy level. We can obtain it by using the periodic table. For example, the elements in group IA of the chemical periodic table have 1 valence electron. electron-dot diagram of an atom of an element in Period 2 of the Periodic Table? ... toothpaste is tin(II) uoride. A town located downstream from a chemical plant was concerned about uoride ions from the plant leaking into its drinking water. According to the Environmental Protection Agency, the Includes Concise Form of Electron Configuration Notation and Tin (Sn). Githy.com. Multiple-site search is loading. Home Money Science & Tech U.S. World Environment Page-of-the-day Trivia Tin (Sn), Electron Configuration: [Kr] 4d 10 5s 2 5p 2 [Kr] 4d 10 5s 2 5p 2::: example of ...
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Oct 22, 1995 · It is also used in the manufacture of super conducting magnets. While tin has many uses in alloys, it has few uses in it's pure elemental form. Additional Notes: Coefficient of linear thermal expansion/K-1 alpha 5.3E-6; beta 21.2E-6. Tin Menu. Tin Page One. Overview of Tin; Tin's Name in Other Languages; Atomic Structure of Tin; Chemical ...
The quick answer here is that because tin, #"Sn"#, is a main-group element, the number of valance electrons will be given by its group number.. Tin is located in group #1color(red)(4)# of the periodic table, which means that it has #color(red)(4)# electrons in its outermost shell, i.e. #color(red)(4)# valence electrons.. Now, you can prove that this is the case by constructing tin's electron ...
In the electron configuration 2-8-7 which value represents the valence electrons? 7 True or false: when drawing an electron dot diagram, you must put the first two electrons next to each other above the element symbol
(or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots ...
atomic symbol. The seventh electron is drawn as a sin-gle dot and is called an unpaired electron. Two fl uorine atoms can share their unpaired electrons and form a covalent bond. We can show this by means of a Lewis diagram as follows: Lets now take an example of an atom with more than one valence electron. The fl uorine atom has seven
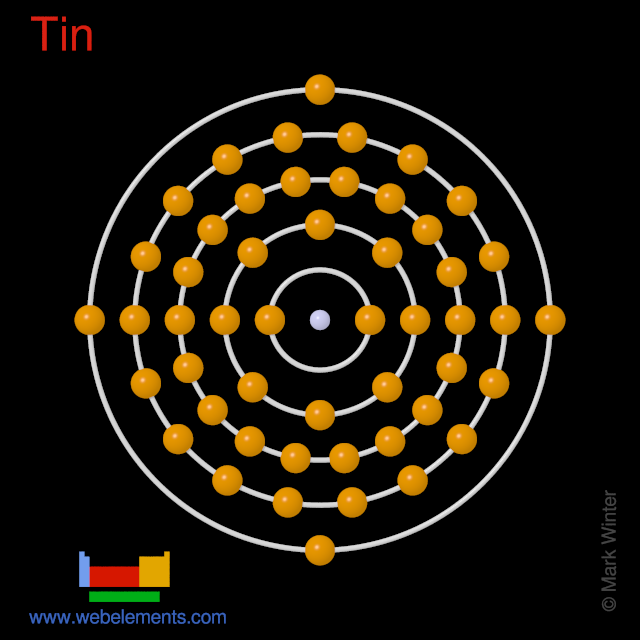
Binary compounds. Compound properties. Element reactions. Tin atoms have 50 electrons and the shell structure is 2.8.18.18.4. The ground state electron configuration of ground state gaseous neutral tin is [ Kr ]. 4d10. 5s2. 5p2 and the term symbol is 3P0. Schematic electronic configuration of tin. The Kossel shell structure of tin.
Since the Lewis electron dot diagrams are based on the number of valence electrons, it would hold true that the elements in the same group would have the same electron dot diagram. In other words, if every element in Group 1A has 1 valence electron, then every Lewis electron dot diagram would have one single dot..... please mark as brainliest.....
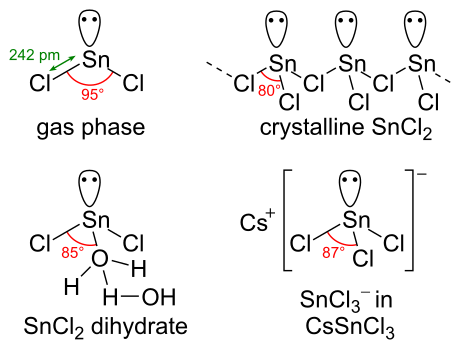
Let's do the SnCl2 Lewis structure. On the periodic table, tin, group 4; and Chlorine, group 7, sometimes called 17, has 7 valence electrons, but we have two of them, so we'll multiply that by two. Four plus 14: 18 total valence electrons. Tin is the least electronegative, goes at the center. Let's put the Chlorines out here on the side.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
A step-by-step explanation of how to draw the SnF2 Lewis Dot Structure.For the SnF2 structure use the periodic table to find the total number of valence elec...
This is the correct dot diagram for sodium, group 1. Q. This is the correct dot diagram for nitrogen, group 15. Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2. Cl, group 17.
Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
A step-by-step explanation of how to draw the P3- Lewis Dot Structure.For the P3- Lewis structure use the periodic table to find the total number of valence ...
Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his article The Atom and the ...
The main uses of stannic chloride are as a raw material for the manufacture of other tine compounds, especially organotins. and in the surface treatmentof glass and other nonconductive materials, whereby stannic oxide is deposited from the stannic chloride solutions onto the surface giving it strength, abrasive resistance, and conductivity. It is also widely used as a catalyst in Friedel-Craft ...
c Dispersive gate charge sensing of a double quantum dot formed under P1 and P2 down to single electron occupancy using the high-impedance TiN resonator as a dispersive gate readout.
A step-by-step explanation of how to draw the Arsenic (As) Lewis Dot Structure.For the ArsenicLewis structure use the periodic table to find the total number...

First Principles Calculations On Bonding Characteristic And Electronic Property Of Tic 111 Tin 111 Interface Sciencedirect

Lychee Like Tio2 Tin Dual Function Composite Material For Lithium Sulfur Batteries Rsc Advances Rsc Publishing

Highly Efficient And Stable Quantum Dot Light Emitting Devices With A Low Temperature Tin Oxide Electron Transport Layer Journal Of Materials Chemistry C Rsc Publishing

Sodium 99 8 Oiled Sticks Wrapped In Aluminium Foil Acros Organics 1kg In Aluminum Foil Packed In Resealable Tin Can Sodium 99 8 Oiled Sticks Wrapped In Aluminium Foil Acros Organics Fisher Scientific
















0 Response to "39 tin electron dot diagram"
Post a Comment