38 diagram of energy states and transitions in the hydrogen atom
Problem: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the ...1 answer · Top answer: [readmore]We are asked to match the responses with the correct arrow.Emission is a transition process from a higher energy level to a lower energy ... The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the longest wavelength._____ 2.) The absorption line with the longest wavelength.____ 3.) The emission line with the lowest energy._____ 4.)
Answer to: The following is a diagram of energy states and transitions in the hydrogen atom. By signing up, you'll get thousands of step-by-step...1 answer · Top answer: Emission spectra are observed when electrons return to the ground state from an excited state. Absorption spectra are observed when an electron goes ...
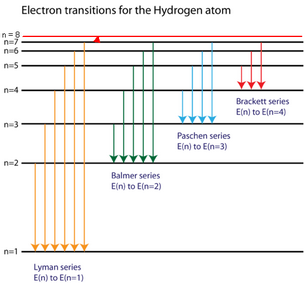
Diagram of energy states and transitions in the hydrogen atom
Question: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from ... The following is a diagram of energy states and transitions in the hydrogen atom. The energy level of the electron of a hydrogen atom is given by the following formula where n denotes the principal quantum number. Energy level diagrams and the hydrogen atom. The absorption line with the shortest wavelength. The followmg is a diagram of energy states and transitions in the hydrogen atom. n = n = Infinity EJ, n B A n = 2 D ENERGY n = 1 Match each of the responses below with the correct arrow from the figure. 4 1 5 1.) The emission line with the longest wavelength 2.) The absorption line with the shortest wavelength. 3.)
Diagram of energy states and transitions in the hydrogen atom. hydrogen atom? We CANNOT measure individual energy levels! We ARE ABLE TO measure indirectly the transition of an electron from one state to another. Absorption and emission of photons. PHYS 1493/1494/2699: Exp. 7 - Spectrum of the Hydrogen Atom The following is a diagram of energy states and transitions in the hydrogen atom. n = infinity n = 4 n = 3 2 n = 2 4 5 ENERGY n = 1 Match each of the responses below with the correct arrow from the figure. a) The emission line with the longest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the highest ... The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon. Lesson Worksheet: Electron Energy Levels. In this worksheet, we will practice determining whether an electron shell of an atom is filled and which electron transitions are possible in a given atom. The diagram shows a hydrogen atom. The electron shown transitions between two energy levels of the atom.
In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are "captured ... For the hydrogen atom, the energy levels only depend on the principal quantum number n. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. Transcribed image text: The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. Transcribed image text: Refer to the energy level diagram for the transitions of the hydrogen atom to answer the following: What is the energy of the principal quantum number 1, in units of reciprocal centimetres, cm-l? Number cm-1 What principal quantum number has an energy of -6855 cm-? Number Referring to the two values above, what amount of energy is given off when an atom transitions from ...
Chemistry questions and answers. The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. The emission line with the longest wavelength. The absorption line with the longest wavelength. The emission line with the highest energy. The absorption line with the highest energy. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy. To conserve energy, a photon with an energy equal to the energy difference between the states will ... The diagram shows the binding energy of each energy level of a hydrogen atom. If an electron is in the ground state, what energy level would it transition to if it absorbed a photon with a wavelength of 97.4 nm? Use a value of 4. 1 4 × 1 0 eV⋅s for the value of the Planck constant. The following is a diagram of energy states and transitions in the hydrogen atom. infinity %3D 4 3 4 ENERGY Match each of the responses below with the correct arrow from the figure. a) The emission line with the shortest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy.
The following is a diagram of energy states and transitions in the hydrogen atom. infinity 1 3 n = 2 ENERGY Match each of the responses below with the correct arrow from the figure. 1.) The emission line with the shortest wavelength. 2.) The absorption line with the longest wavelength. 3.) The emission line with the highest energy. 4.)
This chemistry video tutorial focuses on the bohr model of the hydrogen atom. It explains how to calculate the amount of electron transition energy that is...
This states that the energy scale of hydrogen bound states is a factor of α2 smaller than the rest energy of the electron, that is, about 19000 times smaller. We can thus rewrite the possible energies as: En = −1 2α 2 mc2 1 n. (2.1.6) The typical momentum in the hydrogen atom is p≃ ~ a 0 = me2 ~ = e2 ~c mc → p≃ α(mc), (2.1.7) which ...
A hydrogen atom consists of an electron orbiting its nucleus.The electromagnetic force between the electron and the nuclear proton leads to a set of quantum states for the electron, each with its own energy. These states were visualized by the Bohr model of the hydrogen atom as being distinct orbits around the nucleus. Each energy level, or electron shell , or orbit, is designated by an ...
Question: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from ...
Hydrogen atom quantum numbers A Grotrian diagram is what spectroscopists use to analyze their line spectra. Each column is for a different l quantum number. Note that only certain transitions are observed. These are called selection rules. s p d f g h angular momentum quanta l principal quanta n
The following diagram represents energy levels in a hydrogen atom. Answer Series 2 Answer Series 2 Answer The Transition Labeled For a single electron instead of per mole the formula in ev electron volts is also widely used. The following is a diagram of energy states and transitions in the hydrogen atom. 4 a the emission line with the shortest ...
A hydrogen atom in a state having a binding energy (the energy required to remove an electron) of 0.85 eV makes a transition to a state with an excitation energy (the difference between the energy of the state and that of the ground state) of 10.2 eV.
The 3 d to 2 p transition is a transition from a state with quantum numbers n = 3, l = 2 to a state with n = 2, l = 1. The change in m has to be -1, or 0 or +1. The diagram shows the energy level splitting and the allowed transitions. The different colored arrows show the 3 different photon energies. (c) Because the additional energy due to ...
Electron Transitions The Bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy: This is often expressed in terms of the inverse wavelength or "wave number" as follows: The reason for the variation of R is that for hydrogen the mass of the orbiting electron is not negligible compared to ...
Question: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from the figure. a) The emission line with the ...
The energy level diagram of the hydrogen atom is given below: The transition of electrons from a higher energy level (n>1) to the ground level (n=1) gives rise to the Lyman series in hydrogen spectra.
Figure 22.10 An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. This diagram is for the hydrogen-atom electrons, showing a transition between two orbits having energies E 4 E 4 and E 2 E 2. The energy transition results in a Balmer series line in an ...
Question: The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. The emission line with the longest wavelength. The absorption line with the longest wavelength. The emission line with the highest energy.
The following is a diagram of energy states and transitions in the hydrogen atom. Match each arrow with the correct response below. a) The emission line with the longest wavelength. b) The absorption line with the shortest wavelength. c) The emission line with the lowest energy. d) The absorption line with the highest energy. e) The emission line with the highest frequency.
What are the degeneracies of the Hydrogen atom energy levels? Recall they are dependent on the principle quantum number only. III. Spectroscopy of the Hydrogen Atom Transitions between the energy states (levels) of individual atoms give rise to characteristic atomic spectra. These spectra can be used as analytical tools to assess composition of ...
The followmg is a diagram of energy states and transitions in the hydrogen atom. n = n = Infinity EJ, n B A n = 2 D ENERGY n = 1 Match each of the responses below with the correct arrow from the figure. 4 1 5 1.) The emission line with the longest wavelength 2.) The absorption line with the shortest wavelength. 3.)
The following is a diagram of energy states and transitions in the hydrogen atom. The energy level of the electron of a hydrogen atom is given by the following formula where n denotes the principal quantum number. Energy level diagrams and the hydrogen atom. The absorption line with the shortest wavelength.
Question: The following is a diagram of energy states and transitions in the hydrogen atom. Match each of the responses below with the correct arrow from ...


0 Response to "38 diagram of energy states and transitions in the hydrogen atom"
Post a Comment